Scientists Develop New Method for Peptide Sequencing Based on Nanopore Sensing Technology
New protein sequencing technology with improved sensitivity and throughput will bring revolution to proteomics and clinical diagnostics.
A research team led by Prof. WU Hai-Chen from the Institute of Chemistry of the Chinese Academy of Sciences, and Prof. LIU Lei from the Institute of High Energy Physics of the Chinese Academy of Sciences, together with their collaborators, have developed a new method for peptide sequencing based on host–guest interaction-assisted nanopore sensing.
The study was published in Nature Methods on Nov. 13.
The history of protein sequencing could be dated back to the determination of the complete amino acid sequence of insulin by Sanger in the 1950s. However, so far, there are only two major methods for protein sequencing, i.e. mass spectrometry and Edman degradation. During the past few decades, nanopore sensing emerged as the latest “disruptive” single-molecule technique and has gained great successes in the new generation of DNA sequencing development. This has inspired scientists to transplant the technology to single-molecule protein sequencing. However, nanopore sequencing of proteins faces tremendous challenges, such as the realization of a unidirectional transport of heterogeneously charged peptide chains through a nanopore, and electrical identification of 20 individual amino acids or their combinations.
In this study, the researchers propose an alternative sequencing strategy based on an improved host-guest interaction-assisted nanopore sensing technique. The model peptides are first digested with carboxypeptidases to afford a mixture of amino acids. The next key step is to couple the released amino acids to a FGGCD8ᑕCB[7] peptide probe via a covalent linker and then subject the complex to translocation experiments through wildtype α-hemolysin or its mutants. Finally, the current blockage of each FGGC(X)D8ᑕCB[7] peptide is used to identify the amino acid X and their relative abundance is used to determine the order of the enzymatic cleavage, i.e. the sequence of the peptide.
This serves as a proof-of-concept demonstration for a novel method capable of precisely determining the amino acid sequence of a peptide. While notable limitations persist, this marks a significant advancement and unveils a promising avenue for the future of protein sequencing.
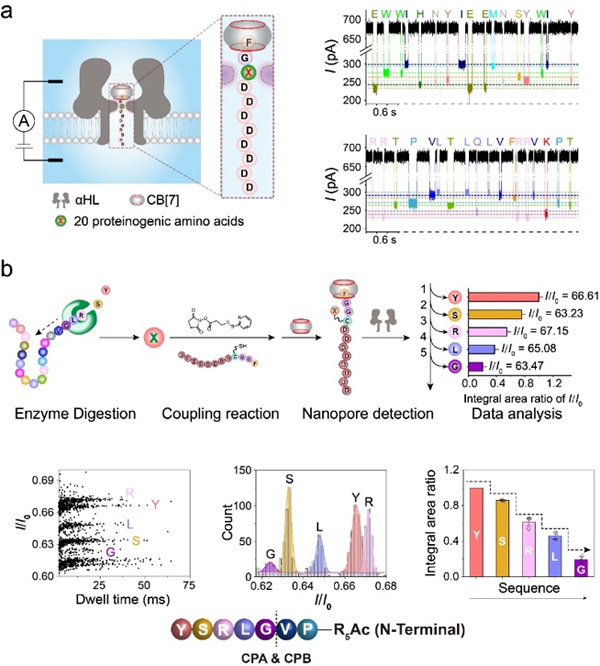
New peptide sequencing method based on host-guest interaction-assisted nanopore sensing. (a) Identification of different amino acids using the FGXD8 probe. (b) The proof-of-concept demonstration of peptide sequencing with the new method. (image by Prof. WU)
Contact:
Prof. WU Haichen
Institute of Chemistry, Chinese Academy of Sciences
Email: haichenwu@iccas.ac.cn





