Solar water splitting is a promising solution to the serious energy crisis in the future. Water oxidation is the bottleneck of overall water splitting. This reaction is a typical proton-coupled electron transfer (PCET) reaction, which involves the transfer of four electrons and four protons. Elucidating water oxidation mechanisms is the key for efficient overall water splitting. Rate law analysis of photo-generated holes have been a powerful tool for the molecular-level understanding of water oxidation mechanisms on semiconductor photocatalysts. For the complete rate law formula of water oxidation, the rate law analysis of H2O is essential, but has seldom been explored for water oxidation on semiconductor photoanodes.
Researchers from the CAS Key Laboratory of Photochemistry of the Institute of Chemistry, Chinese Academy of Sciences have made a series of progress in the photo(electro)catalysis (PEC) water oxidation and revealed the PCET mechanism (J. Am. Chem. Soc. 2016, 138, 2705) and O-O bond formation pathway (J. Am. Chem. Soc. 2018, 140, 3264) of water oxidation on α-Fe2O3 photoanodes. Subsequently, the water oxidation mechanism was further expanded to the oxygen-atom transfer reaction (OAT) (Nat. Catal. 2021, 4, 684-691) and ammonia decomposition reaction (Angew. Chem. Int. Ed. 2022, 61, e202214580), achieving highly selective oxidation of a series of organic and inorganic chemicals.
The PCET reaction can proceed by either a sequential proton-electron transfer (SPET) pathway or a concerted proton-electron transfer (CPET) pathway, which greatly influences the kinetic barrier. Recently, they conducted the rate law analysis of H2O for water oxidation on five well-known photoanodes (i.e., α-Fe2O3, BiVO4, TiO2, Plasmonic Au/TiO2 and n-Silicon) in mixed acetonitrile/water electrolytes by combining with H/D kinetic isotope effect (KIE), electrochemical impedance spectroscopy (EIS) and operando spectroscopic studies. A universal SPET pathway for water oxidation was revealed by the rate law analysis of H2O, which was the rate-limiting factor that caused the sluggish water oxidation performance. It has been the first time that surface modified Ni1-xFexOOH electrocatalyst on photoanodes was found to be a PCET modulator and can induce a mechanistic transition from the SPET to the CPET pathway, significantly enhancing the PEC water oxidation rate. The new mechanistic insights illustrate an effective strategy for modulating PCET kinetics of water oxidation efficient and solar energy conversion on semiconductor surfaces.
The results have been published in the Journal of the American Chemical Society .
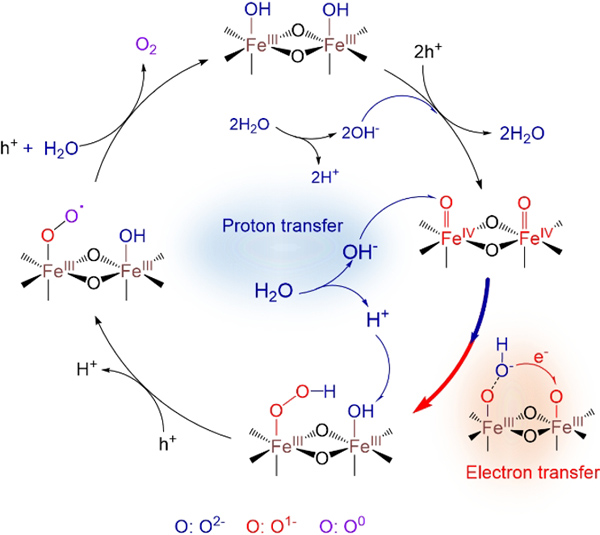
The PCET pathways involved in water oxidation on hematite photoanodes (Image by Prof. ZHANG Yuchao)
Contact:
Prof. ZHANG Yuchao
Institute of Chemistry, Chinese Academy of Sciences
Email: yczhang@iccas.ac.cn