Researchers realize high-efficiency liquid fuel production from carbon dioxide at low temperature
Synthesis of liquid fuel (C5+ hydrocarbons) via CO2 hydrogenation is generally accelerated by heterogeneous catalysts, which involves cascade catalysis of reverse water gas shift (RWGS) reaction to generate CO, and subsequent CO hydrogenation to hydrocarbons via Fischer-Tropsch synthesis (FTS). However, RWGS reaction is endothermic and needs higher temperature, whereas FTS reaction is exothermic and higher temperature tends to inhibit the reaction selectivity. The thermodynamic limitations usually result in high reaction temperature (above 300 oC) and low selectivity of C5+ hydrocarbons.
The homogeneous catalysts are well known for their high efficiency at low reaction temperature. If the RWGS reaction is accelerated effectively at low temperature by a homogeneous catalyst, the above thermodynamic limitations may be overcome. Enlightened by this idea, in a study published in Chem, Prof. HAN Buxing, Prof. QIAN Qingli and co-workers have successfully coupled homogeneous and heterogeneous catalysis in production of liquid fuel from CO2 and H2, where excellent results were achieved at much lower temperature.
The reaction could be effectively catalyzed by homogeneous RuCl3 and heterogeneous Ru0 catalysts in 1-methyl-2-pyrrolidinone (NMP) solvent, where LiCl and LiI were utilized as cocatalyst and promoter, respectively. The reaction was conducted at 180 oC, which is much lower than those reported in the literature. The selectivity of liquid hydrocarbons (C5-C28 n-paraffins) could reach 71.1%, which is the highest to date. The turnover frequency (TOF) of the reaction was as high as 9.5 h-1, which is comparable to the best level of the FTS reaction using Ru0 catalyst. Moreover, the products were all n-paraffins, which has not been reported before in liquid fuel synthesis via CO2 hydrogenation.
The control tests demonstrated that the homogeneous RuCl3 catalyst and the heterogeneous Ru0 catalyst could be recycled and reused, respectively. This suggested that both of them had good stability when they are utilized separately. When they are coupled together in a reactor, the contents of the homogeneous catalytic components (cationic Ru, Li+, Cl- and I-) before and after the reaction were nearly the same, which confirmed the stability of the homogeneous and the heterogeneous catalysts in the consecutive reactions.
Detailed study indicated that synergy of catalytic components and/or the consecutive reactions accounted for the outstanding performance of the catalytic system.
This work opens a new avenue for high-efficient production of liquid fuel via CO2 hydrogenation at milder condition.
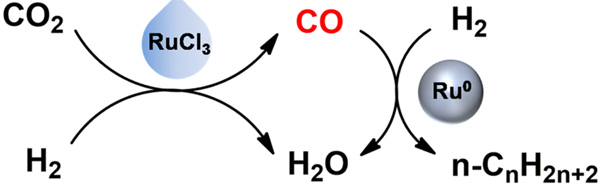
Hydrogenation of CO2 by coupling homogeneous and heterogeneous catalysis (Image by Prof. HAN Buxing).
Contact:
Prof. HAN Buxing
Institute of Chemistry, Chinese Academy of Sciences
Email: hanbx@iccas.ac.cn





