Researchers develop new second near-infrared window fluorescent probe with emission wavelength over 1200 nm.
Researchers develop new second near-infrared window fluorescent probe with emission wavelength over 1200 nm.
Fluorophores in the second near-infrared window (NIR-II, 1000?1700 nm) have attracted extensive attention due to their better imaging quality and deeper tissue penetration than those in the first near-infrared window (NIR-I, 650?900 nm). Currently, NIR-II fluorophores are primarily based on inorganic nanomaterials, but their disputable toxicity hinders preclinical translation. In contrast, organic small molecular fluorophores have presented satisfactory safety profiles and have been used in clinics for years. Therefore, organic fluorophores are preferred for in vivo imaging. So far, there are mainly two types of NIR-II organic small molecular fluorophores, characterized by their structures. One is the polymethines, which however suffer from limited Stokes shifts and poor chemical stability. The other type of fluorophores has the donor-acceptor-donor (D-A-D) feature, and great progress has been made with them recently. A typical example is the benzo[1,2-c:4,5-c]bis([1,2,5]thiadiazole) (BBTD) derivative. Compared to polymethines, the D-A-D fluorophores show larger Stokes shift (>200 nm) and higher signal-to-background ratio for in vivo imaging. However, the maximal absorption and emission wavelengths of the existing BBTD-based fluorophores for in vivo imaging are located at about 800 and 1000 nm, respectively, which are still relatively short. Thus, developing new fluorophores with longer wavelengths is highly demanded.
Recently, Dr. Shi Wen and Dr. Fang Yu in the research team led by Prof. Ma Huimin (Huimin Ma) from Institute of Chemistry, Chinese Academy of Sciences (CAS), have developed a new small organic molecular fluorophore, FM1210 (see Figure below), with maximal emission of 1210 nm, and compared its spectral properties and image quality with those of the S-substituted analogue, CF1065. Remarkably, FM1210 displayed a large redshift of 145 nm in emission wavelength with comparable quantum yield and brightness. This largely increased wavelength beyond 1200 nm may be ascribed to the synergistic contribution of both the Se atom and the amino group, thereby enabling in vivo imaging using FM1210 to be of much higher quality than that with CF1065. Also, the FM1210 encapsulated in liposomes can be applied to the in vivo imaging of the malignant tumor and its vasculature with a high signal-to-background ratio.
The study entitled “Design, Synthesis, and Application of a Small Molecular NIR-II Fluorophore with Maximal Emission beyond 1200 nm” was published in J. Am. Chem. Soc. (2020, 142, 15271-15275). The research was supported from the National Natural Science Foundation of China, and Youth Innovation Promotion Association of CAS.
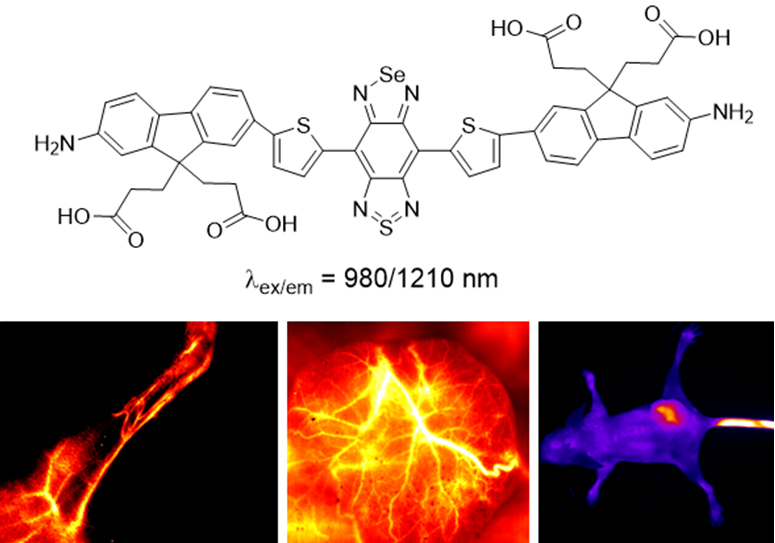
The structure of FM1210 and its fluorescence imaging in vivo (image by Prof. MA Huimin).
Contact:
Prof. MA Huimin
Institute of Chemistry, Chinese Academy of Sciences
Email: mahm@iccas.ac.cn





