Researchers Observe New Isotope Effect in the Vacuum Ultraviolet Photodissociation of Carbon Monoxide
Carbon monoxide (CO) is the second most abundant molecular species just after H2 in the interstellar medium. The photolysis properties of CO in vacuum ultraviolet (VUV) region are believed to play important roles in explaining the anomalous C and O isotope distributions observed in the Solar System. These isotope distributions contain key information of the evolution history of the Solar System. It has long been known that rare isotope (13C, 17O, 18O) substitution can substantially change the absorption line positions, oscillator strengths and photodissociation rates of CO, while how the isotope substitution changes the photodissociation products has never been explored before, which can greatly affect the subsequent chemical reactions.
Recently, a research group from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS), led by Prof. GAO Hong, reported that the isotope substitution can greatly alter the amounts of electronically excited C and O atoms produced in the VUV photodissociation of CO. The work entitled “Strong and selective isotope effect in the vacuum ultraviolet photodissociation branching ratios of carbon monoxide” was published in Nature Communications (DOI: 10.1038/s41467-019-11086-z).
To perform this study, researchers excite the two isotopomers 12C16O and 13C16O to the same rovibronic quantum state by absorbing a single VUV photon, and accurately measure the kinetic energies of the photofragments by using a home-built time-slice velocity-map ion (TS-VMI) imaging setup, from which the relative ratios between C(3P) [or O(3P)] in the ground state and C(1D) [or O(1D)] in the excited state generated in the photodissociation process are deduced. The study finds that these ratios strongly depend on the isotope substitution, as shown in the figure. This could potentially affect the subsequent chemical reactions that happen in the interstellar medium, since C and O atoms in the ground state hardly react with H2, the most abundant species in the universe, due to the extremely low temperature in the interstellar space; while the reactions of C and O atoms in the excited state with H2 are barrierless, thus could proceed with substantial rates to form hydrocarbons and water even at very low temperatures.
This work was financially supported by the National Natural Science Foundation of China, Beijing National Laboratory for Molecular Sciences and ICCAS.
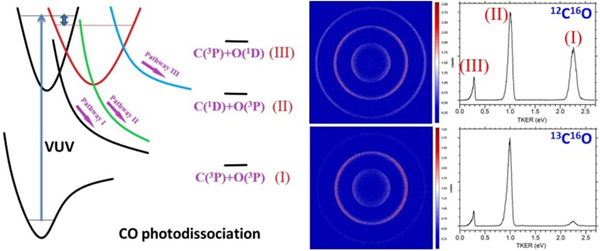
Isotope effect of the photodissociation branching ratios of CO (Image by Prof. GAO Hong)
Contact:
Prof. GAO Hong
Institute of Chemistry, Chinese Academy of Sciences
Email: honggao2017@iccas.ac.cn





