Scientists Design Mn-Based Heterogeneous Catalyst with Halogen and Nitrogen Dual-Coordination Towards Highly Efficient Electrocatalytic Reduction of CO2
The excessive consumption of fossil with excessive emission of CO2 has triggered serious energy supply shortage and environmental issues. Electrochemical carbon dioxide (CO2) reduction reaction (CO2RR) with water as reaction medium is a promising approach for both decreasing atmospheric CO2 concentration and producing value-added carbon products. Common heterogeneous catalysts are metals, particularly nanostructured noble metals such as Au, Ag and Pd, which have shown to be active for CO2RR in aqueous solution at low overpotentials. However, the low abundance and high cost of these precious metals limits their large-scale application. It is much desirable to develop highly efficient and selective heterogeneous electrocatalysts for CO2RR based on cheap and earth-abundant metals.
Recently, ZHANG Bingxing and Prof. ZHANG Jianling from Institute of Chemistry, Chinese Academy of Sciences and their collaborators reported that the Mn-based heterogeneous catalyst can be efficient and selective electrocatalyst for converting CO2 to carbon monoxide (CO) via halogen and nitrogen dual-coordination to modulate the electronic structure of Mn atoms. Outstandingly, the CO faradaic efficiency (FECO) is up to 97% with a current density of ~10 mA cm-2 at a low overpotential of 0.49 V. Moreover, the turnover frequency (TOF) for CO2RR can reach 38347 h-1 at overpotential of 0.49 V, which outperforms all the reported heterogeneous electrocatalysts under similar conditions. The in situ synchrotron X-ray absorption spectra (XAS) for CO2RR and theoretical simulations reveal the promoted CO2 activation process and facilitated CO desorption process. This study opens up an opportunity for improving the CO2RR properties of metal electrocatalysts under mild conditions, particularly for the metals with intrinsically low activity.
This work supported by National Natural Science Foundation of China, Ministry of Science and Technology of China and the Chinese Academy of Sciences. The EXAFS experiment was conducted at Beijing Synchrotron Radiation Facility, and the result was published in Nat. Commun. (2019, 10, 2980).
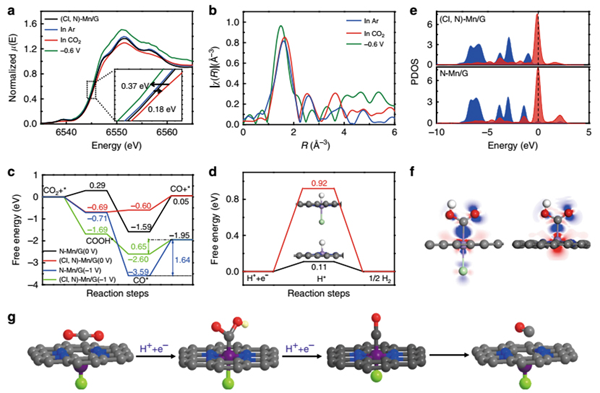
In situ XAS experiment and DFT calculation.(Image by ZHANG Bingxing and Prof. ZHANG Jianling)
Contact:
Prof. ZHANG Jianling
Institute of Chemistry, Chinese Academy of Sciences
E-mail: zhangjl@iccas.ac.cn





