Researchers Develop Mild and Efficient Lithium Recycling Strategy from Spent Lithium-ion Batteries
With the electrification of transportation and the transformation of the energy landscape, lithium-ion batteries (LIBs), as a sustainable clean energy storage device, have undergone a prosperous period of development. However, the expanding production of LIBs has simultaneously led to a shortage of essential raw materials, particularly lithium. The recycling of LIBs, especially lithium, is crucial for addressing resource scarcity, minimizing environmental impact, promoting a circular economy, and enhancing energy efficiency. It is an essential step towards achieving a more sustainable and responsible approach to battery production and usage.
In a study published in Angewandte Chemie, the research group led by Prof. GUO Yuguo and MENG Qinghai from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) proposed a novel lithium recycling strategy. They use potential controllable redox couples to mildly and effectively extract lithium from spent batteries. The research has been featured as newsworthy and highlighted by the editorial office of Wiley (Efficient and mild: recycling of used lithium-ion batteries).
Compared to the inherent idea of recycling lithium from spent cathodes, extracting it from the anodes (typically graphite) is more efficient as it eliminates the need for separating transition metals. However, the leaching process for lithiated anodes using water as a solvent is accompanied by the release of a significant amount of heat and the escape of flammable and explosive gases. In this study, polar aprotic solutions consist of a polycyclic aromatic hydrocarbon (PAH) and an ether as the solvent were astutely chosen as the extraction agent. This selection helps prevent the occurrence of excessive heat generation and the evolution of hydrogen gas. Benefiting from the tunable electron affinities and tailored potential of PAHs, Li+-electron concerted redox reactions between anodes and PAH solutions proceeds spontaneously, leading to effective lithium extraction (achieving close to 100% leaching efficiency) and anode deactivation.
Besides, the Li-PAH solutions produced can serve as direct lithiation reagents. For example, the regeneration of degraded LiFePO4 (LFP) cathode was realized by mixing powder and PAH solution at room temperature. The regenerated LFP delivered a discharge capacity of 143.5 mAh g-1 at activation stage and demonstrated cycle stability similar to that of pristine LFP.
This work reduces the safety risks, mitigates waste and hazards, and achieves perfect atomic economy during the recycling process. It serves as a source of inspiration for designing innovative solutions to meet the demands of safely and sustainably recycling surplus energy batteries.
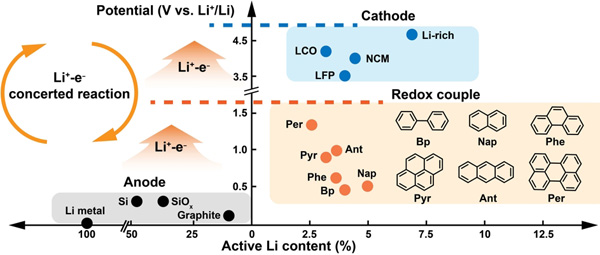
Potential controllable redox couple for mild and efficient lithium extraction from anodes (Image by CHANG Xin).
Contact:
Prof. GUO Yuguo
Institute of Chemistry, Chinese Academy of Sciences
Email: ygguo@iccas.ac.cn





