Hydrogen-bonded Organic Frameworks Facilitate Intracellular Enzyme Catalysis
Enzyme catalysis within cells not only enables in-situ monitoring of cellular metabolism for disease diagnosis, but also offers promising therapeutic benefits for defective enzyme-induced diseases. Hydrogen-bonded organic frameworks (HOFs) is well suited to serve as a general platform for encapsulation of enzymes to facilitate intracellular biocatalysis
In a study recently published in Angewandte Chemie International Edition(DOI: 10.1002/anie.202105634), the research group led by Prof. Ming Wang from Institute of Chemistry, Chinese Academy of Sciences (ICCAS) developed a universal strategy for enzyme incorporation into nanoscale HOFs through in-situ self-assembly of proteins and HOFs precursors, which further showed preserved enzymatic activity inside living cells.
In this work, the researchers firstly prepared nanosized HOFs via self-assembly of tetrakis(4-amidiniumphenyl)methane (T) and azobenzenedicarboxylate (A) in aqueous solution at room temperature termed as TA-HOFs. Furthermore, by optimization of the concentration of HOFs motifs and proteins, they successfully obtained nanoscale protein-encapsulated biocomposites, which showed preserved protein structure and function. The as-prepared protein@TA-HOFs can further deliver protein into cells without scarfing protein function. They used green fluorescent protein (GFP) as a proof of concept study. GFP@TA-HOFs showed high uptake efficiency by human cervical carcinoma (HeLa) cells with minor cellular toxicity. In addition, efficient endo/lysosome escape was also confirmed by counterstaining of endosome. Notably, the in-situ self-assembly strategy was generally applicable for proteins with different surface charges and sizes to prepare nanoscale pretein@biocomposites. Moreover, TA-HOFs can efficiently deliver both the highly surface charged GFPs, further proving the universal of the TA-HOFs cellular delivery platform.
Intracellular delivery of enzymes was also studied for enzymatic catalysis inside cells. They found that TA-HOFs can deliver enzymes such as horseradish peroxidase (HRP) and β-galactosidase into HeLa cells with preserved enzyme activity. Furthermore, intracellular delivery of catalase (CAT) into neural cells (SH-SY5Y) can alleviate reactive oxygen species (ROS) accumulation inside cells, which is one of the causes of most neurodegenerative diseases.
This study paved the avenue for applying HOFs as a novel universal platform for biomacromolecule delivery and disease treatment, and highlighted in Nature Reviews Chemistry.
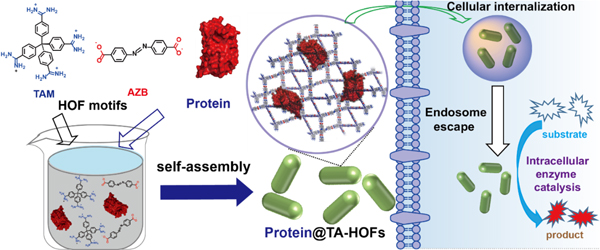
In situ self-assembly and encapsulation of proteins into nanoscale HOFs for intracellular biocatalysis (Image by Prof. WANG)
Contact:
Prof. WANG Ming
Institute of Chemistry, Chinese Academy of Sciences
Email: mingwang@iccas.ac.cn





