Researchers Reveal Dynamic Evolution of Cathode Interphase in Quasi-Solid-State Lithium Batteries
Solid-state lithium batteries (SSLBs) have been widely considered as an increasingly valuable frontier for the next generation of energy storage devices because of their high security and electrochemical stability. However, the inferior chemical compatibility between the cathode and electrolyte leads to poor battery performance during cycling. The surface evolution of cathodes and their interphase can directly influence the electrochemical reactions of the electrode and thus the battery degradation mechanisms.
Recently, the research group led by Prof. WEN Rui at CAS Key Laboratory of Molecular Nanostructure and Nanotechnology, Institute of Chemistry of the Chinese Academy of Sciences (ICCAS), have made progress in understanding the surface mechanism of dynamic evolution of chemical and mechanical properties of cathode interphase in Quasi-Solid-State Lithium Batteries (QSSLBs).
In this study, the scientists used in-situ electrochemical atomic force microscopy (EC-AFM) to directly visualize the real-time evolution of both surface morphology and mechanical properties of the cathode interphase on NCM523 particles at the nanoscale upon charging/discharging. The live formation of cathode interphase with inorganic-organic hybrid structure was real-time elucidated, as well as the evolution of its mechanical property by in-situ scanning of the Derjaguin-Muller-Toporov (DMT) modulus mapping.
Furthermore, the chemical components in cathode interphase layer were identified in detail at different stages of cycling by the X-ray photoelectron spectroscopy (XPS). It is discovered that LiF-rich species first form at 4.08 V on charge, followed by the formation of organic species such as ROLi and ROCO2Li. Subsequently, inorganic components including Li2CO3 and LiF deposit on the surface of electrode at 3.4 V on discharge. The organic and inorganic compositions in cathode interphase could exhibit different mechanical properties.
They further confirmed that the phase transformation from R 3m (rhombohedral phase) to Fd 3m (spinel phase) and Fm 3m (rock-salt) occurs on grain boundaries of NCM523 particle during cycling. The phase reconstruction could inhibit Li+ transport and further aggravate the degradation of the battery. The researchers further found that the cathode interphase layer has a significant impact on the electrochemical behavior of QSSLBs, and a relatively stable cathode interphase layer would help to improve the electrochemical performance.
This study throws light on surface mechanisms of the cathode interphase on NCM523 cathodes and offers guidance for the design of SSLBs with superior performance.
This work entitled "Dynamic Evolution of a Cathode Interphase Layer at the Surface of LiNi0.5Co0.2Mn0.3O2 in Quasi-Solid-State Lithium Batteries" was published in Journal of the American Chemical Society (2021, DOI: 10.1021/jacs.0c09602).
The research work was financially supported by the Ministry of Science and Technology of China and National Natural Science Foundation of China.
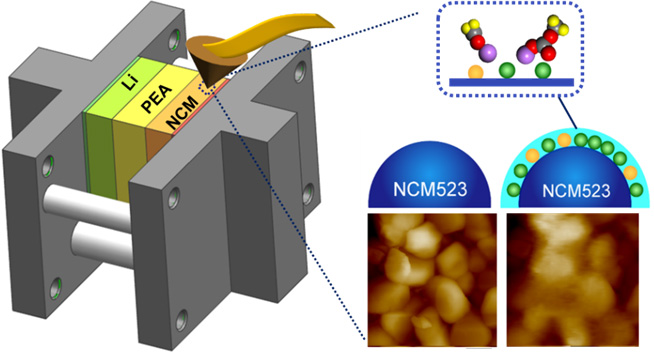
Schematic representation of the QSSLB for in-situ AFM imaging and surface evolution of cathode interphase layer (Image by Prof. WEN's group)
Contact:
Prof. WEN Rui
Institute of Chemistry, Chinese Academy of Sciences
Email: ruiwen@iccas.ac.cn





