Researchers Develop Mesopolymers Synthesis by Ligand-modulated Direct Arylation Polycondensation
Conjugated polymers offer tremendous opportunities for semiconductor devices with solution-processability and mechanical flexibility, such as field-effect transistors. However, the emergence of structural defects, low solubility and batch-to-batch variation in conjugated polymer synthesis hamper their high-quality electronic applications. As an intermediate between small molecules and polymers, mesopolymers possess medium chain sizes and well-defined structure are anticipated to address these limitations.
Recently, a joint research group from the Institute of Chemistry of Chinese Academy of Sciences (ICCAS) and Tianjin University, led by Prof. HU Wenping, reported that the synthesis of mesopolymers by ligand-modulated direct arylation polycondensation for high performance n-type and ambipolar semiconducting materials. The work entitled “Mesopolymer synthesis by ligand-modulated direct arylation polycondensation towards n-type and ambipolar conjugated systems” was published in in Nature Chemistry.
Researchers initiated their study thorough ligand screening in direct arylation polycondensation of DPP and 4,7-dibromobenzo[c][1,2,5]thiadiazole (BTz). Di(1-adamantyl)-n-butylphosphine (Ad2PnBu) is demonstrate to be more efficient than other commercial available ligands to afford meso-DPPBTz with low dispersity and suitable chain lengths. The steric effect of Ad2PnBu is decelerate the chain growth process to avoid undesired units dislocation in the main chain. Hence, major side reactions such as homocoupling and β-arylation defects are expected to be minimized by their protocol. As a proof, NMR, MALDI–TOF–MS and DFT results validate the strictly alternating structure without detectable homocoupling and β-arylation defects in meso-DPPBTz. Due to the medium chain length, mesopolymers’ bandgaps are widened while their lowest unoccupied molecular orbitals are shifted downwardly. As a result, electron mobilities in mesopolymers outperform their polymer counterparts with a magnitude of 1.7 to 124 fold times.
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, National Natural Science Foundation of China, and Ministry of Science and Technology of China.
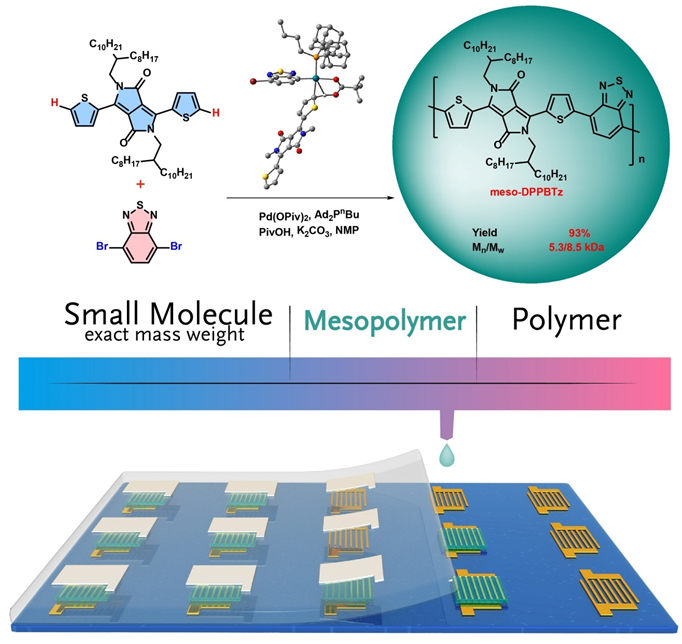
synthesis of mesopolymers via DArP. (Image by Prof. HU Wenping)
Contact:
Prof. DONG Huanli
Institute of Chemistry, Chinese Academy of Sciences
Email: dhl522@iccas.ac.cn





