Scientists Develop a New spectroscopic probe and its use in fluorescence imaging of monoamine oxidase A
Spectroscopic probes may be described as the reagents that can interact with analytes (targets) accompanied by the changes of their spectroscopic (absorption, or luminescent) properties, and based on such spectroscopic changes the analytes can thus be determined. Spectroscopic probes have been extensively investigated and widely used in many fields because of their powerful ability to improve analytical sensitivity, and in particular to offer greater temporal and spatial capability for in vivo imaging studies. Therefore, developing new spectroscopic probes and specific imaging methods are important for various biological studies.
Prof. MA Huimin’s research group at the Institute of Chemistry, Chinese Academy of Sciences, has been engaged in this field for more than two decades, during which a series of new spectroscopic probes and sensing/labeling methods for biologically active species have been developed by employing different chemical reactions. Based on their outstanding achievements in the field, Prof. Ma and his colleagues have been invited to summarize various design strategies on spectroscopic probes (Chem. Commun., 2012, 48, 8732-8744; Chem. Rev., 2014, 114, 590-659; Chem. Sci., 2016, 7, 6309-6315).
Monoamine oxidase (MAO) exists in two isoforms, MAO-A and MAO-B. However, they play different roles in bioprocesses, for example, MAO-A instead of MAO-B preferentially decomposes serotonin. Therefore, specific imaging of the two isomers in cells is crucial to further reveal their biofunction. At present, fluorescent probes selective for MAO-B have been reported, but developing the probes specific for MAO-A is still of great challenge. Moreover, propylamine, the traditional recognition moiety of MAO, can be oxidized by both MAO-A and MAO-B in some cases, causing the cross interference. In order to address this issue, the researchers at ICCAS noticed the fact that clorgyline is a specific inhibitor of MAO-A, whereas pargyline is that of MAO-B; the only major difference between the two inhibitors is the substituted phenol moiety. Based on this, Prof. Ma and his colleagues have recently proposed a general design strategy for a fluorescent probe specific for an enzyme by using the characteristic structure of the enzymatic inhibitor, and this has been successfully demonstrated with MAO-A. Namely, they prepared a series of resorufin-derivatized fluorescent probes by combining the substituted phenol of clorgyline with propylamine as a new recognition moiety for MAO-A. All these probes can effectively recognize MAO-A over MAO-B. Moreover, the representative probe has been used to image the endogenous MAO-A in live cells such as SH-SY5Y and HepG2 with different levels of MAO-A by confocal fluorescence imaging. The superior specificity of the probe for MAO-A has been further validated by western blot assay, control probe and cell transfection experiments. Notably, the proposed strategy may be useful to design specific fluorescent probes for other enzymes (Angew. Chem. Int. Ed.,2017, 57, 15319-15323).
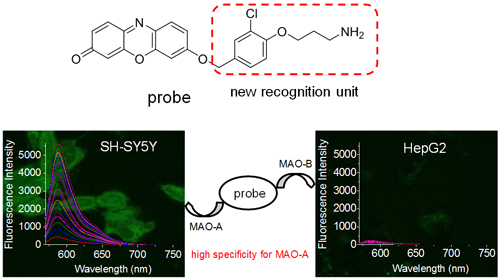
New spectroscopic probe and its use in specific fluorescence imaging of MAO-A.(Image by Prof. MA Huimin)
Contact:
Prof. MA Huimin
Institute of Chemistry, Chinese Academy of Sciences
Email: mahm@iccas.ac.cn





