Scientists Reveal the Interfacial Process and Mechanism in Lithium–Sulfur Batteries
Li–S batteries are one of the most promising energy storage systems owing to their high theoretical energy density (2600 Wh kg-1), low cost, and the abundance of sulfur. However, several critical problems for Li–S batteries, such as the polysulfides (PS) shuttling and insulating nature of sulfur, hinder their practical application. To achieve a significant breakthrough, it is urgently needed for the mechanistic understanding of interfacial reaction and fundamental electrochemistry of Li-S batteries.
Via in situ electrochemical atomic force microscopy (EC-AFM), Prof. WAN Lijun and Prof. WEN Rui from Institute of Chemistry of Chinese Academy of Sciences (CAS) investigated the dynamic nucleation, growth, deposition, and dissolution processes of insoluble Li2S2 and Li2S at the cathode/electrolyte interface in Li/PS semi-liquid batteries at the nanoscale.
The monitored interfacial dynamics show that Li2S2 nanoparticle nuclei begin to grow at 2 V followed by a fast deposition of lamellar Li2S at 1.83 V on discharge. Upon charging, only Li2S depletes from the interface, leaving some Li2S2 undissolved, which accumulates during cycling. It is indicated that the cathode passivation during cycling is mainly caused by the accumulation of insoluble Li2S2 sediments.
To investigate the relationship between the interfacial reaction and system performance, the voltage-capacity curves were measured under galvanostatic modes with different current rates. At high current rates, large over-potential is beneficial for the growth of Li2S and leads to the rapid deposition. Lower rates enable the slow discharge of PS and increase intermediate reactions, at which only Li2S2 nanoparticles can be observed at the interface.
These findings reveal a straightforward structure–reactivity correlation and performance fading mechanism in Li–S batteries. The work was published in Angew Chem Int Ed.
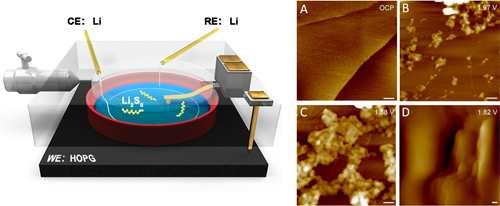
The nanoscale processes at a highly oriented pyrolytic graphite (HOPG) cathode/polysulfide-electrolyte interface were directly observed by using in situ AFM. The morphology and growth processes of insoluble products Li2S2 and Li2S were monitored and provided evidence for a mechanism for capacity fading in Li-S batteries. (Image by Prof. WEN Rui)
Contact:
Prof. WEN Rui
Key Laboratory of Molecular Nanostructure and Nanotechnology,
Institute of Chemistry,Chinese Academy of Sciences
Email: ruiwen@iccas.ac.cn





