Great Progress on Lithium-Sulfur Batteries
Under the supports of the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, the Chinese Academy of Sciences, and Bosch Investment Ltd., Prof. Yu-Guo Guo’s group from the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology made great progress on the development of high-performance sulfur cathode materials by using smaller sulfur molecules for lithium-sulfur batteries. The results have been published in J. Am. Chem. Soc., (2012, 134, 18510−18513) , and highlighted by Chemical & Engineering News entitled with “High-Energy Battery Built To Last” (http://cen.acs.org/articles/90/web/2012/11/High-Energy-Battery-Built-Last.html )。
The lithium-sulfur battery holds a high theoretical energy density, 4-5 times that of today’s lithium-ion batteries, yet its applications have been hindered by poor electronic conductivity of the sulfur cathode and, most importantly, the rapid fading of its capacity due to the formation of soluble polysulfide intermediates (Li2Sn, n = 4-8).
Despite numerous efforts concerning this issue, combatting sulfur loss remains one of the greatest challenges. Researchers from ICCAS, IoP, and Bosch Research & Technology Center show that this problem can be effectively diminished by controlling the sulfur as smaller allotropes.
They have successfully realized the metastable sulfur allotropes S2−4 via confining them in conductive carbon micropores (Figure 1). These confined small S2−4 molecules exhibit a high Li electroactivity and a novel electrochemical behavior with a single output plateau at -1.9 V, in contrast to the common cyclo-S8. The results demonstrated that the confined S 2-4 as a new cathode material can totally avoid the unfavorable transition between the commonly used large S8 and S42- , and essentially solve the critical problem of polysulfide dissolution in conventional Li−S batteries. The as-obtained S2−4 in S/(CNT@MPC) show a high specific capacity of 1670 mA·h/g, an impressive cycling stability of 1150 mA·h/g after 200 cycles, and a favorable high-rate capability of 800 mA·h/g at 5 C (Figure 2). The success of the novel S cathode promises a new Li−S battery with higher energy density (785W·h/kg based on anode and cathode) than state-of-the-art Li-ion batteries (theoretically 387 W·h/kg in a LiCoO2/C battery) for applications in portable electronics, electric vehicles, and large-scale energy storage systems.

Figure 1. Schematic illustration of the smaller sulfur molecules for better lithium−sulfur batteries. (Image by GUO Yuguo)
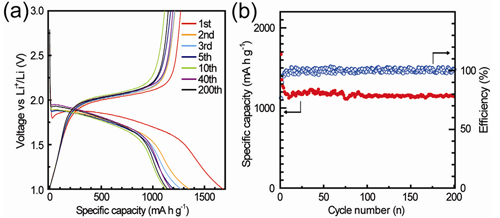
Figure 2. Electrochemical properties of the small sulfur/carbon cathode material. (a) Discharge/charge voltage profiles at 0.1 C. (b) Cycling performance at 0.1 C. (Image by GUO Yuguo)





