ICCAS Researchers Make Series of Progress on Asymmetric Primary Amine Catalysis
Inspired by the exquisite stereocontrol in the enzymatic enamine processes, Prof. Luo Sanzhong of the Institute of Chemistry, the Chinese Academy of Sciences(ICCAS) and co-workers developed a novel type of synergetic primary-tertiary diamine organocatalysts in the past few years. This simple organocatalyst functionally imitates six different natural aldolases and could be successfully applied to the asymmetric aldol reactions of a whole range of ketone donors, such as aliphatic ketones, cyclic ketones, hydroxyacetone, dihydroxyacetone, pyruvic derivatives, acetacetic derivatives and acetaldehyde (J. Am. Chem. Soc. 2007, 129, 3074-3075; Org. Lett. 2008, 10, 653-656; Org. Lett. 2008, 10, 1775-1778; J. Org. Chem. 2009, 74, 1747-1750; J. Org .Chem. 2009, 74, 9521-9523; J. Org. Chem. 2010, 75, 4501–4507; Eur. J. Org. Chem. 2011,3347–3352.). In a manner closely resembling its enzymatic counterpart, this small molecule could also catalyze stereoselective retro-aldol reactions with surprisingly efficacy. These interesting features have enabled an unprecedented asymmetric transfer-aldol reaction, i.e. an forward direct aldol and retro aldol in one pot. (Chem. Eur. J. 2010, 16, 4457-4461; Org. Biomol. Chem. 2011, 9, 1784–1790.)(Scheme 1)

Scheme 1. Bio-inspired primary-tertiary diamine catalyst(Image by Luo Sanzhong et al.)
Further investigation revealed that asymmetric supramolecular catalysis could be realized via covalently immobilization of primary amine catalysts on supramolecular hosts such as β-cyclodextrin. The catalytic aldol reaction in aqueous buffer was found to follow Michealis-Menten kinetics, thus presented the first example of asymmetric supramolecular catalysis under enzymatic conditions, which nicely bridges small molecular catalysis and enzymes. Meanwhile, the detailed mechanism study provided some useful hints for the development of new generation of bio-inspired primary amine catalysts (J. Am. Chem. Soc. 2010, 132, 7216–7228).
Enantioselective protonation is one of the most straightforward approaches for building α-chiral carbonyl compounds in nature and in synthetic chemistry. However, the majority of methods rely on protonation of a prochiral enolate, the protonation of enamine intermediate through enamine catalysis has remained an elusive goal. In their continuing efforts, Luo and co-workers realized the primary amine catalyzed enantioselective Friedel-Crafts -protonation reaction. Unlike the previous reported enantioselctive Friedel-Crafts process, α-substituted acrolein was used as electrophile and no chiral center was formed during the Carbon-Carbon bond formation step, the only chiral center was generated during the protonation of the enamine intermediate. It is found that the primary-tertiary diamine catalyst could efficiently manipulate the protonation process with high enatio-control, mechanism study revealed that the real proton source in this reaction is the in-situ formed or extra added H2O, in addition, the OH…π interaction in the protonation transition state between H2O and indole ring played an important role in the enantioselectivity (Angew. Chem. Int. Ed., 2011, 50, 11451-11455) (Scheme 2).
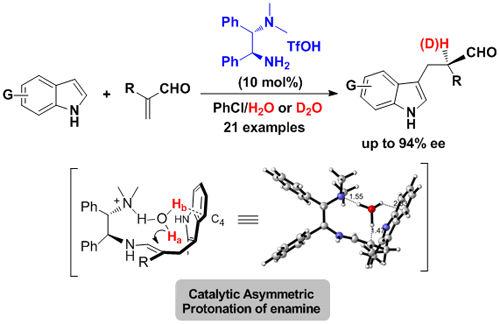
Scheme 2. Primary-Tertiary diamine catalyzed enantioselective protonation(Image by Luo Sanzhong et al.)





