N-heterocyclic Carbene-Catalyzed Enantioselecitve Synthesis of Sulfur-Containing Compounds
Sulfur-heterocycles are key motifs for wide varied bioactive compounds, and chiral sulfur-containing molecules find many applications in asymmetric synthesis. The catalytic asymmetric synthesis of sulfur-heterocycles and related compounds is of great value and highly desirable.
Under the financial support from Ministry of Science and Technology, the National Natural Science Foundation of China and the Chinese Academy of Sciences, chemists at the ICCAS developed the N-heterocyclic carbene (NHC)-catalyzed asymmetric synthesis of chiral sulfur-containing molecules. Based on the previous reported NHC-catalyzed reaction of ketenes (Org. Lett. 2008, 10, 277; Angew. Chem. Int. Ed. 2009, 48, 192; Angew. Chem. Int. Ed. 2010, 49, 8412),the NHC-catalyzed enantioselective formal [2 + 2] cycloaddition of N-sufinylannilines and ketenes was developed, giving the corresponding sulfur-heterocycles in good yields with high diastereo- and enantioselectivities. Furthermore, oxidation or reductive ring opening the cycloadduct afforded the 3-oxo-beta-sultams, alpha-mercapto acid derivatives and beta-mercapto amines (Angew. Chem. Int. Ed. 2011, 50, 9104).
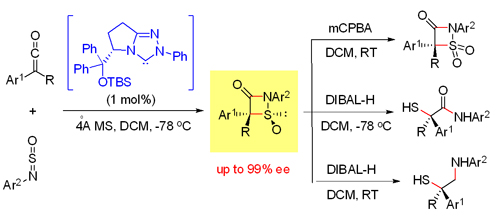
NHC-catalyzed synthesis and applications of thiazetidinone oxide(Image by YE Song)





