Porphyrin Supramolecular Nanostructures: Controlled Assembly and Supramolecular Chirality
Owing to their unique architectures, tailored physicochemical properties etc., organic nanomaterials with tunable morphologies have attracted great interest in the interdisciplinary areas of nanoscience, supramolecular chemistry, and soft matter. Among various building blocks, porphyrins have been considered to be one of the most important components for the assembly of organic nanostructures due to their aromatic electron delocalization over the molecular frame, unique planar as well as rigid molecular geometry, which render them with peculiar and tunable spectroscopic, photophysical, photochemical, and assembly properties. Thus far, various porphyrin-involved functional nanostructures have been manufactured by means of several sophisticated methods, where the surfactant-assisted self-assembly (SAS) technique have been received particular attention. In a general SAS process, organic units, which are dissolved in a guest solvent, are assembled with the assistance of surfactants that are dispersed in a host solvent. Commonly, the host and guest solvents have nice compatibility. Taking into account of the nice solubility of organic units in apolar or low-polar medium, a SAS using an oil/water system might be a subject of general interest.
Recently, researchers from Institute of Chemistry, Chinese Academy of Sciences (ICCAS) have found that a Zinc porphyrin (ZnTPyP, see picture) could be controlled assembled to form diverse nanostructures via a SAS by using an oil/water system as medium (J. Am. Chem. Soc.2010,132, 9644–9652). They have demonstrated that when a chloroform (oil) solution of ZnTPyP is added dorpwise to a CTAB aqueous solution (water), hollow nanospheres, solid nanospheres, nanotubes, nanorods and nanofibers could be facilely synthesized depending on the concentration of the CTAB aqueous solution and the aging time. Moreover, the synthesized nanorods could be further hierarchically organized to form regular nanoarray over large-area solid supports, while the others could not. Interestingly, although the employed ZnTPyP and CTAB species are achiral compounds, distinct supramolecular chirality could be observed from the nanorods, but could not from the other nanostructures. On the basis of their experiment facts, the researchers have proposed a tentative explanation for these interesting phenomena. Taking account into the general interest of porphyrin and CTAB, the method might open up a general way for the controlled synthesis of organic nanostructures. In their previous works, researchers from this group have systematically investigated the mirror symmetry breaking occurred at the air/water interface (J. Am. Chem. Soc.2003,125,5051;J. Am. Chem. Soc.2004,126, 1322;J. Am. Chem. Soc.2009,131, 2756;Chem. Eur. J.2008,14, 1793), the present study suggest that symmetry breaking phenomenon could also occur at the liquid/liquid interface, and it sheds new lights on the chirogenesis process in porphyin-involved supramolecular systems. More intensive works on this subject are now underway by these researchers.
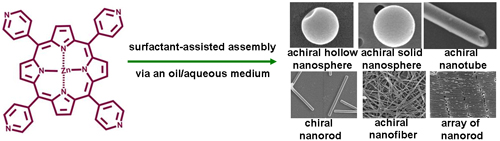
ZnTPyP could be controlled organized to form various well-defined nanostructures by means of a SAS, where an oil/water system is employed as medium





