A Highly Efficient Route for Selective Phenol Hydrogenation to Cyclohexanone
Cyclohexanone is a key raw material in the synthesis of many useful chemical intermediates, such as caprolactam for nylon 6 and adipic acid for nylon 66. The phenol hydrogenation is one of the main routes for producing cyclohexanone. However, the attainment of high selectivity at elevated conversion with a satisfactory rate is a great challenge, because the cyclohexanone product can be further hydrogenated to cyclohexanol under the reaction conditions.
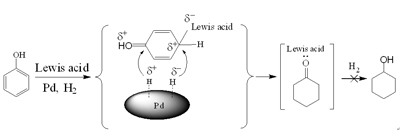
With the financial support of National Natural Science Foundation of China, the Ministry of Science and Technology of China, and the Chinese Academy of Sciences, the researchers at the Institute of Chemistry, Chinese Academy of Sciences found that common commercial palladium catalysts (supported on carbon, alumina, or NaY zeolite) and a Lewis acid such as aluminum chloride could synergistically promote this reaction, and a preliminary mechanism was proposed on the basis of kinetic and spectroscopic studies. A Lewis acid itself could not catalyze the reaction, but it could make the benzene ring of phenol more active. At the same time, acid-base interaction between the Lewis acid and cyclohexanone inhibits further hydrogenation to cyclohexanol. The conversion and selectivity could approach 100% simultaneously under mild conditions, and therefore all the phenol could be converted into the product and the separation process was simple. Further study demonstrated that the reaction conducted in compressed CO2 was more efficient and environmentally benign, and the reaction efficiency could be tuned by controlling the phase behavior of the reaction system.
Science,2009, 326, 1250-1252