Methane, one of the most abundant raw materials from fossil and biogenic sources, constitutes an important feedstock for the synthesis of major energy sources. Because of the highly strong C-H bonds, activation and the further transformation of methane into value-added chemicals is usually performed under extreme conditions, such as high temperatures and high pressures. Furthermore, it is of particularly challenging to uncover the elementary steps and mechanism details in methane transformation, the chemical processes of which are fundamentally interesting and practically important. Therefore, the activation of methane under mild conditions has attracted great scientific studies for decades.
Atomic clusters that can be handled under isolated conditions are being actively studied for activation of methane. The positively charged clusters are very reactive with methane than the corresponding negatively charged species, particularly for noble metal free anions. Recently, a research group at Institute of Chemistry, the Chinese Academy of Sciences (ICCAS) headed by Prof. HE Sheng-Gui reported that the reactivity of molybdenum carbide cluster anions toward methane can be much enhanced by the adsorption of a CO molecule. This work was published on Angew. Chem. Int. Ed..
The isolated Mo2C2– cluster is inert with CH4 molecule. Mass spectrometry, photoelectron spectroscopy and quantum chemistry calculations demonstrated that Mo2C3O– cluster with the dissociated attached CO molecule is much more reactive. In sharp contrast, the Mo2C3O– isomer with the non-dissociated CO molecule is unreactive toward CH4. For the first time, the noble-metal-free cluster anions (Mo2C3O–) that can react with CH4 thermal collision conditions has been reported. The dissociative adsorption of CO molecule on Mo2C2– cluster can tune down the spin density distribution on one Mo atom, and then the activation of CH4 was promoted through oxidative addition.
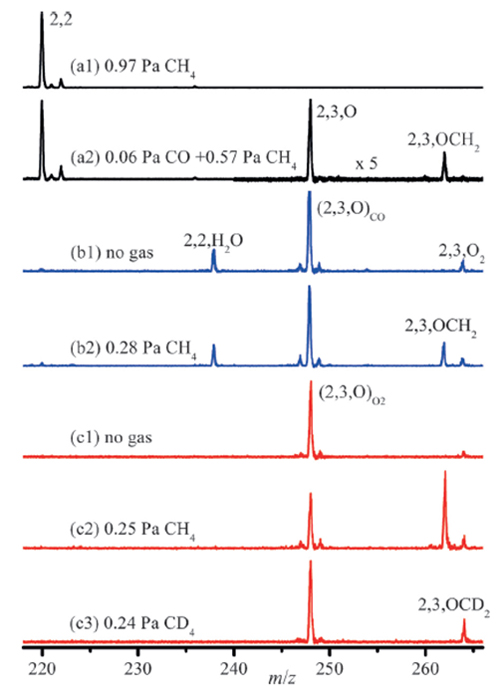
Cluster reactivity: Time-of-flight mass spectra of the reactions of mass-selected 98Mo2C2–(Image by HE Shenggui)
Contact:
Prof. HE Shenggui
The Laboratory for Structural Chemistry of Unstable Species ,
Institute of Chemistry, Chinese Academy of Sciences
Email: shengguihe@iccas.ac.cn
