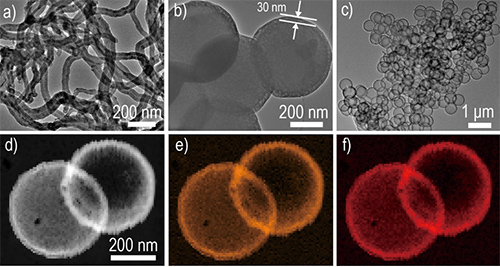
Core–shell structures have attracted a great deal of interest due to their versatility in regulating different components towards diverse applications such as catalysis, biology, energy conversion, and energy storage. As far as the coating shells are concerned, it has been a well-known challenge to construct different metal oxide nanoshells, not to mention a precise control on the uniformity and shell thickness of the surface coatings.
With the supports from the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, the Chinese Academy of Sciences, the research group from the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology made a series of achievements in surface modification of cathode materials in lithium ion batteries. Different synthetic protocols have been developed to achieve a precise control of the surface properties. Firstly, for those polyanion-type cathode materials which have a low electron conductivity, different in-situ polymerization processes have been developed to achieve a thin polymer coating layer on the surface, which would be turned into conductive carbon from a following carbonization process. In this way, the rate capability and cyclability of the polyanion-type cathode materials can be obviously improved. Meanwhile, the carbon nanoshell also lead to much safer cathode materials as revealed by the less heat released and iron dissolved during cycling. (RSC Adv., 2014, 4, 7795-7798 and J. Mater. Chem. A, 2014, 2, 17359-17365). Secondly, for cathode materials such as LiCoO2, a full protection by metal oxides, typically Al2O3 have been highly expected but hardly achieved due to the synthesis challenge. Prof. An-Min Cao and his co-workers propose to use buffer solution as a specific reaction media for Al2O3 coating, which produces uniform Al2O3 nanoshells with their thickness controlled at one nanometer precision. The application of this methodology for the surface modification of LiCoO2 shows that the battery performance can be successfully optimized through a surface control on the Al2O3 nanoshells.
Angew. Chem. Int. Ed. 2014, 53, 12776 –12780)