A microemulsion is a thermodynamically stable dispersion of two immiscible fluids stabilized by surfactants. Owing to the capacity to simultaneously host a variety of polar and nonpolar species, microemulsions have been widely applied in protein delivery, drug release, catalysis, and nanomaterial synthesis. In general, the organic solvent (oil) and water are used as the two immiscible fluids in the formation of microemulsions. In recent years, supercritical CO2 (SCCO2) and ionic liquids (ILs), considered as unconventional and green solvents, have received much attention in different fields.
Under the supports of the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, and the Chinese Academy of Sciences, the researchers in the CAS Key Laboratory of Colloid and Interface and Thermodynamics from Institute of Chemistry recently reported the creation of CO2-in-IL microemulsions(Angew. Chem. Int. Ed., 2011, 50, 9911-9915). This novel kind of microemulsion has many advantages. For example, the size of the dispersed CO2 droplet can be tuned by the pressure of CO2; the properties of the continuous phase can also be tuned by the kind of ILs due to the tunable and designable features of ILs. These special properties make CO2-in-IL microemulsion have different applications in material synthesis, chemical reaction, extraction, etc.
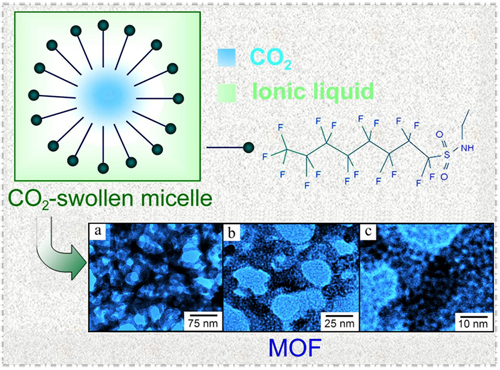
Carbon Dioxide-in-Ionic Liquid Microemulsions(ZHANG Jjianling)
