Development of sensors and receptors for biologically important anions is a hot topic of current interest because of their indispensable roles in vital (or physiological) processes. Among the anions, fluoride is one of the most attractive targets for its considerable significance to health and environmental issues. As an essential element of body for normal physiological functions, the optimal level of 1 mg/day or 2-4 mg/L of consumed fluoride is affirmed by EPA.
At present, the ion selective electrode method, the ion chromatography method and the standard Willard and Winter method are almost universal approaches for fluoride quantitative analysis. Meanwhile these methods also introduce more or less disadvantages, such as complicated procedures, high cost or low mobility. Funded by National Natural Science Foundation of China, researchers from Institute of Chemistry, Chinese Academy of Sciences develop a rapid and portable sensing method for aqueous fluoride ion with two independent modes of signal transduction based on fluoride-dependent changes of fluorescence color.
In the study, the researchers silylate an ESIPT (excited state intramolecular proton transfer) compound 3-BTHPB to afford a derivative BTTPB. The BTTPB is dispersed into CTAB/water micelle and fabricated as a stable sensor system for fluoride ion in water. After addition of fluoride ion, fluorescence color of the sensor show a gradual change from violet to bright yellow, and the change is accurately fluoride-dependent. To facilitate the use of the system, test paper for the fluoride ion detection based on the BTTPB system is prepared. According to the following figures, it can be easily observed if the fluoride ion concentration in drinking water exceeds the standard.
This research result is published in Angew. Chem. Int. Ed. 2010, 49, 4915-4918.
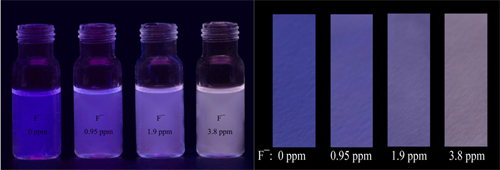
Figure 1. Pictures of BTTPB dispersions/test paper upon addition of NaF
