Lithium batteries, as a main power source or back-up power source for mobile communication devices, portable electronic devices and the like, have attracted much attention in the scientific and industrial fields due to their high electromotive force and high energy density. To meet the demand for batteries having higher energy density and improved cycle characteristics, in recent years, a great deal of attempt has been made to develop new electrode materials or design new structures of electrode materials.
With the financial supports of the Chinese Academy of Science, MOST and the National Natural Science Foundation, researchers of the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology, have made a progress in V2O5 hollow microspheres for high-performance cathode materials in lithium-ion batteries (Angew. Chem. Int. Ed. 2005, 44, 4391-4395). More recently, they successfully designed and synthesized tin-based nanostructured anode materials for high-performance lithium-ion batteries. The work has been published in the recent issue of Adv. Mater. (2008, 20, 1160-1165), and has been highlighted by the Nanowerk (http://www.nanowerk.com/spotlight/spotid=5210.php) .
Metallic tin is considered to be a very promising anode material for lithium-ion batteries because its theoretical specific capacity (Li4.4Sn, 992 mAh/g) is much higher than that of conventional graphite (LiC6, 372 mAh/g). However, the biggest challenge for employing metallic tin as applicable active anode materials is that it is suffering from huge volume variation during the lithium insertion/extraction cycle, which leads to pulverization of the electrode and very rapid capacity decay. In the present work, they therefore designed a new approach to synthesize tin nanoparticles encapsulated elastic hollow carbon spheres (TNHCs) with uniform size, in which multiple tin nanoparticles with a diameter of less than 100 nm were encapsulated in one thin hollow carbon sphere with a thickness of only about 20 nm, thus leading to both the content of Sn up to over 70% by weight and the void volume in carbon shell as high as about 70–80% by volume. This void volume and the elasticity of thin carbon spherical shell can efficiently accommodate the volume change of tin nanoparticles due to the Li-Sn alloying-dealloying reactions, and thus prevent the pulverization of the electrodes. As a result, this type of tin-based nanocomposites have very high specific capacity of >800 mAh/g in the initial 10 cycles, and 550 mAh/g after the 100th cycle, as well as excellent cycling performance, exhibiting a great potential as anode materials in lithium-ion batteries.
Advanced Materials, 2008, 20, 1160-1165.
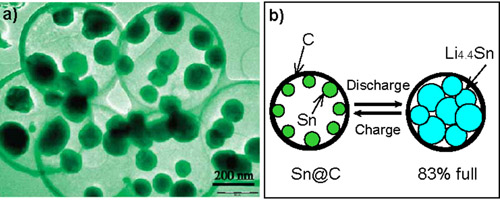
Figure 1. The structure of the Sn@C composites: (a) The TEM image; (b) The scheme of the insertion/extraction of Li+ during discharge/charge processes.
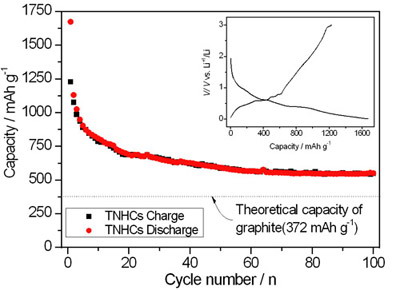
Figure 2. The discharge/charge voltage profiles of Sn@C composites, the inset shows the first discharge/charge profiles of the composites.
, 