| |
Ionic liquids (ILs) are a kind of ionic media with unusual properties such as extremely low vapor pressure, wide liquid temperature range and electrochemical window, and strong ability to absorb microwave, etc, which make them unique solvents for many promising applications. In their previous work, prof. Zhimin Liu and coworkers successfully deposited precious metal nanoparticles on natural clays with the aid of guanidinium-based ionic liquids, and the resultant composites exhibited excellent catalytic activities and stability for hydrogenations of benzene and alkenes (Angew. Chem. Inter. Ed., 2006, 45(2), 266-269;J. Phys. Chem. C. 2007 111(5) 2185-2190.).
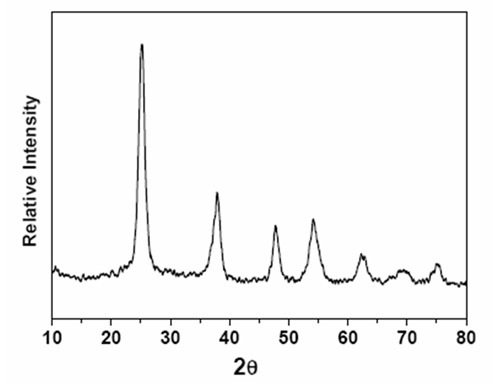
More recently, with financial supports of the National Natural Science Foundation and Chinese Academy of Science, they developed a method to prepare metal oxide nanocrystals via microwave heating IL solution dissolving metal precursor. For instance, high quality TiO2 nanocrystals were controllably synthesized using 1-butyl-3-methylimidazolium tetrafluoroborate as medium and titanium isopropoxide as precursor. As illustrated in Figures, the resultant nanocrystals display uniform particle size and regular shape. Similarly, other metal oxide nanocrystals (e.g, SnO2) also could be synthesized. This method is simple and fast, in which IL acted as reaction medium as well as structure inducing agent.
J. Am. Chem. Soc., (2007, 129, 6362-6363.)

 , |
|
