Inspired by the exquisite stereocontrol in the enzymatic enamine processes in which natural enzymes such as type I aldolases use the primary amine of a lysine moiety to form the enamine intermediate chemists have long been pursuing simple chemical mimic for asymmetric enamine catalysis. This goal has now been accomplished by a research group of assistant professor Sanzhong Luo and adjunct professor Jin-Pei Cheng in ICCAS. The researchers developed a series of simple chiral primary-tertiary diamine catalysts such as trans-N N-diaminocyclohexanes. In the presence of a strong acid such as CF3SO3H the diamines catalyzed direct asymmetric aldol reactions at room temperature in isolated yields as high as 99% and enantiomeric excesses up to >99%.. Significantly the reactions accommodate the synthetically important but challenging substrates such as linear aliphatic ketones such as butanone with high regioselectivity and unprecedented syn diastereoselectivity. These results are in sharp contrast with the secondary amine mediated similar reactions wherein anti diastereoselectivity were normally observed.
J. Am. Chem. Soc.(2007 Vol. 129 No. 11 p. 3074-3075)
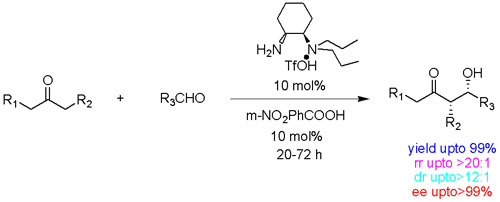
A: Picture for Reaction Model
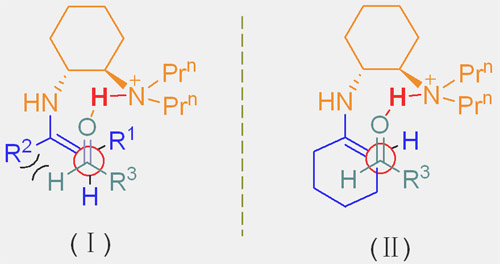
B: Transition state: (Ⅰ) Linear ketone (Ⅱ) cyclic ketones.
, |
