| |
With supports from the Chinese Academy, Natural Science Foundation of China, and the Minsitry of Science and Technology, the researchers in the Key Laboratory of Molecular Nanostructures and Nanotechnologies have made significant advances in elucidating the mechanism of methanol to olefin conversion, using the so called “shape selectivity” of the zeolites.
Methanol to olefin (MTO) process is important to chemical industry as well as the economy. It provides a independent route other than crude oil for providing basic feed stock materials for petroleum chemical industry. The mechanism of the reaction, though, is still elusive. The main difficulty for the researchers is the secondary reactions during the MTO process, when primary products, ethylene and propene, quickly convert to other hydrocarbons, making it almost impossible to observe the primary product. The research team, led by Professor Weiguo Song and Professor Li-Jun Wan, took advantage of the shape selectivity of the zeolite pores and synthesized zeolites with desired pore structures, which enabled the researchers to control the size of the organic intermediate during the MTO process, so that the reaction could proceed to certain degree, but could finish the whole catalytic cycle, e.g. no olefin was produced, so the secondary reactions were eliminated. The researchers were able to observe isotope exchange from the organic intermediate to study the mechanism of C-H bond activation in methanol.
The study was recently published in the Angewandte Chemie and was named as the VIP articles.
Angew. Chem. Int. Ed. ( 2006, vol. 45, 6512-6515 )
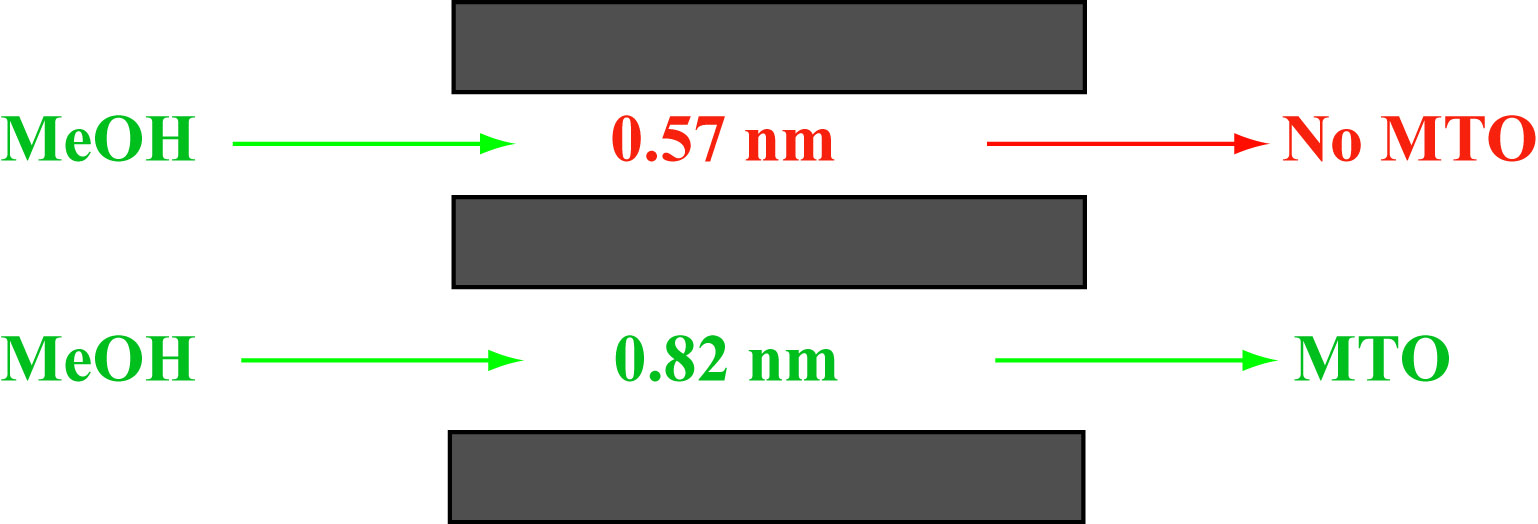
Methanol is converted to olefins on zeolite with a 0.82 nm pores; while on zeolite with 0.57 nm pores, it is not. , |
|
