An-Min Cao Jin-Song Hu Han-Pu Liang Li-Jun Wan and Chun-Li Bai
Self-assembled structures with highly specific morphology and novel property are of great interest to chemists and materials scientists. Controlled organization from rod-like building blocks to curved structures remains a challenge. Such a capability is attractive to scientists not only because of its importance in understanding the concept of self-assembly with unnatural building blocks but also for its great application potentials.
Vanadium pentoxide (V2O5) has been extensively studied as a well-known transition-metal oxide. The nanostructured V2O5 shows great potential applications in the field of lithium-ion batteries actuators catalysis and sensors. A mediated-polyol process is then adopted to synthesize highly ordered superstructure of vanadium pentoxide (V2O5) in which nanorods circle around leading to hollow microspheres in various shapes from “nest” to “hedgehog”. The protocol represents a substantial simplification over more conventional methods such as electrostatic spray or thermal evaporation. The state of nanorods on the surface is readily tunable by changing the concentration of V(acac)3. The formed microspheres can adopt various shapes from a nest to a hedgehog. The prepared V2O5 exhibits desirable electrochemical properties in terms of high capacity at remarkable reversibility when it is used as cathode material in lithium-ion battery.
Angew. Chem. Int. Ed. 2005 Vol 44 No. 28 4391-4395
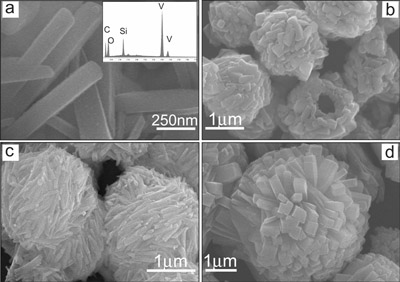
a) SEM image of the prepared vanadium precursor with 6 mM V(acac)3 and no PVP. The inset is the EDX pattern of the rod. b c d) SEM images of the microspheres synthesized with different concentrations of V(acac)3 at b) 6 mM c) 18 mM and d) 42 mM while the concentration of PVP was constant at 0.14 mM.
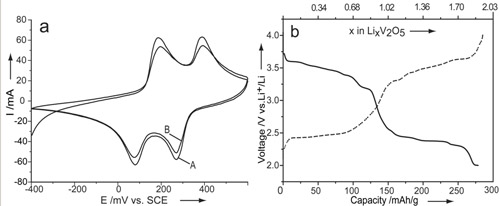
a) Cyclic Voltammograms of V2O5 microspheres obtained at a scan rate of 10 mV/s. First scan is indicated by line A and after 15 cycles by line B. b) The third charge (dash-dot line)-discharge (solid line) curve with the current rate of C/5. The capacity was as high as 286.4 mAh/g while the charge-discharge efficiency was up to the level of 97.2% in the potential range from 2.0 V to 4.0 V. , |
