Mei-Xiang Wang* and Hai-Bo Yang
A number of aza- and/or oxo-bridged calix[2]arene[2]triazines have been synthesized through an unusually high yielding and efficient fragment coupling approach starting from cyanuric chloride and resorcinol 3-aminophenol m-phenylenediamine and NN’-dimethyl-m-phenylenediamine. These novel macrocycles which belong to the next generation of calixarenes or cyclophanes form a unique cavity that is resulted from two isolated benzene planes and two bis-heteroatom-conjugated triazine planes in a 13-alternate fashion. The nature of the bridging heteroatoms i.e. combination of the electronic conjugative and steric effects of the nitrogen and oxygen atoms strongly regulates the cavity size generating a set of fine-tuned cavities in which the distance between two benzene rings at the upper rim ranges from 5.011 Å to 7.979 Å. The multiple intermolecular hydrogen bond interactions among NN’-dimethylated tetraazacalix[2]arene[2]triazines and among tetraazacalix[2]arene[2]triazines leads to the formation of infinite one dimensional chain structure and two dimensional zigzag layered structure respectively in the solid state. The ease of preparation and further chemical manipulations and the readily tunable cavity structures render these aza- and/or oxo-bridged calix[2]arene[2]triazines the unique platforms in the study of supramolecular chemistry.
J. Am. Chem. Soc. 2004 126 15412-15422
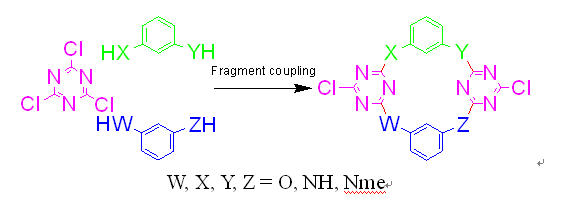
A general and high yielding fragment coupling synthesis of heteroatom-bridged calixarenes
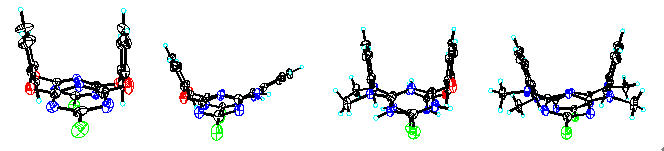
Selected examples of fine-tuned heteroatom-bridged macrocycles
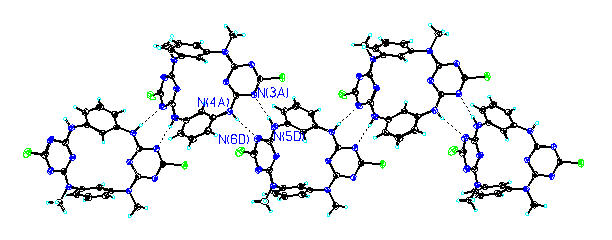
One dimensional chain structure formed through intermolecular hydrogen bonds in the solid state. , |
