Relayed Electron Transfer Mediated by Imidazolium Radical
Prof. KANG Xinchen and Prof. HAN Buxing’s group at the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) developed an ionic liquid (IL) 1-ethyl-2,3-dimethylimidazolium nitrate (EmmimNO3) anchored bismuth catalyst (IL@Bi) and for the first time, proposed a “relayed electron transfer” mechanism, which enabled the efficient electrochemical synthesis of glycine from oxalic acid and nitrate/air.
The electrochemical synthesis of amino acids from oxalic acid and nitrate/air represents a frontier in green chemistry and sustainable synthesis. Glycine has wide applications in the pharmaceutical, food, and chemical industries. However, the electrosynthesis process involves complex electron–proton transfer processes, and the key intermediate, glyoxylic oxime (GAO), exhibits sluggish kinetics, leading to low selectivity and yield.
At −1.3 V versus reversible hydrogen electrode, the catalyst achieves a Faradaic efficiency of 81.1% for glycine and a high current density of 286.2 mA cm-2, outperforming pure Bi electrodes and previously reported catalysts. In addition, the research group uses nitrogen oxides (NOx) generated from plasma-activated air as the nitrogen source, achieving a glycine selectivity of up to 89.0%. This demonstrates the feasibility of producing high-value amino acids directly from abundant air, highlighting the low cost, green, and sustainable nature of the feedstock under this strategy.
Mechanistic studies indicate that the excellent performance of the IL@Bi catalyst arises from its unique “relayed electron transfer” mechanism. During electrolysis, Emmim+ on the Bi surface is reduced to Emmim• radicals, which act as efficient electron mediators, rapidly transferring electrons to the key intermediate GAO and promoting the formation of highly reactive GAO• radicals. This significantly enhances the kinetics of GAO conversion to glycine. The mechanism bypasses the slow direct electron transfer from Bi to GAO, while the IL modification effectively suppresses the hydrogen evolution reaction, collectively enabling a highly efficient conversion.
Other ILs, such as 1-ethyl-2,3-dimethylimidazolium bromide (EmmimBr), N-ethyl-pyridinium nitrate (EpyNO3), and 1-octyl-2,3-dimethylimidazolium nitrate (OmmimNO3), are introduced to mediate the electron transfer process, all of which effectively facilitate the electron transfer processes. Under the promotion of this mechanism, the use of various carbon sources enables the efficient synthesis of different α-amino acids, demonstrating the generality of the IL-mediated “relayed electron transfer” mechanism.
This study not only developed a high-performance catalyst for the electrochemical synthesis of glycine but also revealed a new electron transfer mechanism mediated by IL radicals. It provides a novel strategy for designing efficient and general electrocatalytic C–N coupling systems.
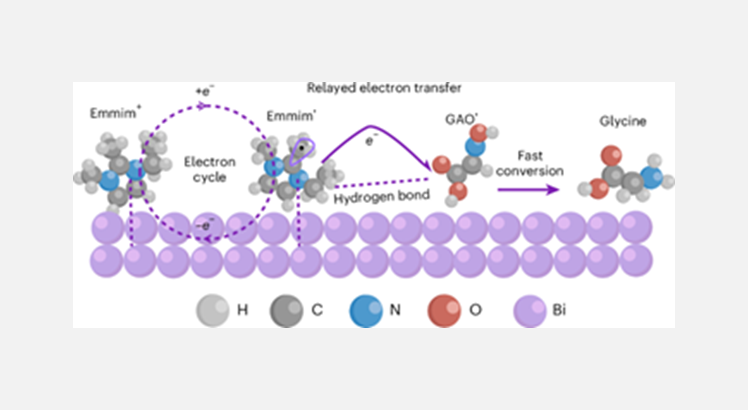
Relayed electron transfer mediated by imidazolium radical
(Image by WANG Hengan)
This study was published in Nature Synthesis.
Contact:
Prof. KANG Xinchen and Prof. HAN Buxing
Institute of Chemistry, Chinese Academy of Sciences
Email: kangxinchen@iccas.ac.cn; hanbx@iccas.ac.cn





