Researchers Develop Praseodymium-Copper Oxides for Efficient CO2 Electroreduction
Prof. HAN Buxing and Prof. ZHU Qinggong’s group from the Institute of Chemistry, Chinese Academy of Sciences developed a facile strategy to establish Pr-Cu oxide heterointerfaces for efficient CO2 electroreduction, achieving selective production of C2+ alcohols.
CO2 is a greenhouse gas that contributes to climate change. But what if we could capture it and turn it into something useful? Electrochemical CO₂ reduction reaction (CO₂RR) powered by renewable electricity is a key approach to transforming CO2 into high-value chemicals. Among the potential products, multi-carbon alcohols (e.g., ethanol, n-propanol) hold great promise for clean energy storage and as chemical feedstocks, thanks to their high energy density and compatibility. However, a major challenge in electrocatalytic CO₂RR for C₂⁺ alcohol synthesis is the competition with reactions that produce ethylene in the C₂⁺ pathway. Developing new high-efficiency catalysts to enhance the selectivity of C₂⁺ alcohols is thus crucial for progress in this field.
The researchers fabricated a special catalyst made from Pr6O11-Cu through ‘stepwise precipitation and calcination method’, which means they gradually added and heated the materials in stages. This process created tiny, uneven active sites with lots of different interfaces, these engineered interfaces were identified as key catalytic centers for CO₂ activation and conversion.
The catalyst exhibited excellent activity and selectivity for CO2 reduction to C2+ alcohols. At -1.08 V vs RHE, the current density and faradaic efficiency (FE) of C2+ alcohols could reach 700 mA cm-2 and 71.3%, respectively, with the FEs of ethanol and n-propanol reaching 58.6% and 12.7%, respectively. The C2+ alcohols/C2H4 ratio was an impressive 12/1.
Further investigation revealed that the Pr⁴⁺/Pr³⁺ redox pair interacts synergistically with Cuδ⁺/Cu⁰ via Pr-O-Cu linkages, forming self-healing Pr-Ovac-Cu interfaces. This unique architecture not only ensures structural stability but also induces charge redistribution that modulates *CO intermediate adsorption. The resulting mixed adsorption configurations on asymmetric sites promote a typical C-C coupling while stabilizing alcohol intermediates, thereby dramatically enhancing C2+ alcohols selectivity.
“Our study provides a route for designing efficient catalysts towards C2+ alcohols production in CO2RR, and may trigger the rational design of novel rare-earth-based catalysts for efficient CO2 electrolysis.” Said Prof ZHU.
This study was published in Nature Synthesis.
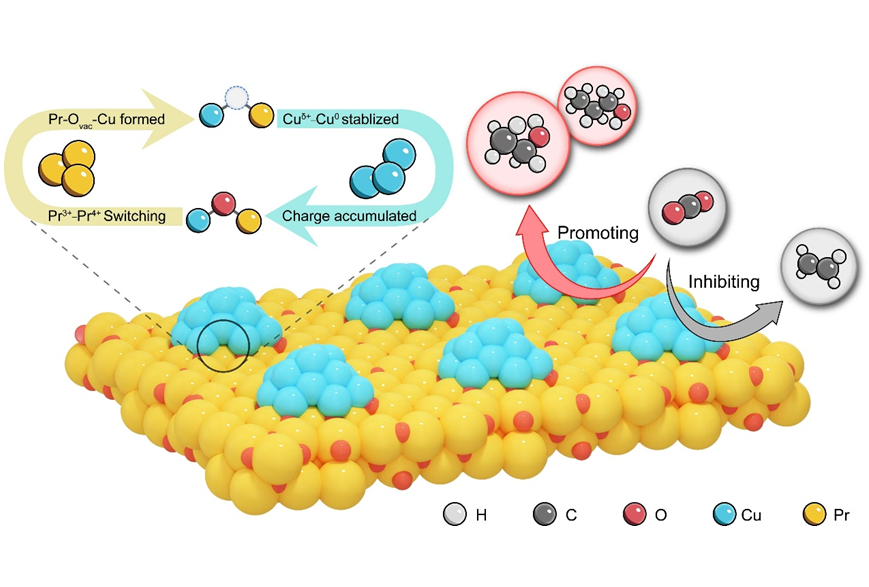
Electrocatalytic CO2 hydrogenation to C2+ alcohols over Pr-Cu oxide heterointerfaces. (Image by LIU Jiyuan)
Contact:
Prof. ZHU Qinggong, Prof HAN Buxing
Institute of Chemistry, Chinese Academy of Sciences
Email: qgzhu@iccas.ac.cn; hanbx@iccas.ac.cn





