Researchers Develop Versatile and Efficient Oxygen Atom Transfer Reactions in Photoelectrochemical Cells
Photo(electro)catalysis has attracted heightened interest in environmental remediation and solar energy conversion. However, those widely studied photo(electro)catalysts (e.g., TiO2) work via single-electron transfer pathways, which generate active radicals for driving photochemical reactions. For environmental remediation, these active radicals degrade target pollutants as well as other coexisting organics (e.g., humic acid) unselectively, which causes a low efficiency towards target pollutants. This unselectivity also hinders the development of photo(electro)catalysis for selective organic synthesis.
Researchers at the CAS Key Laboratory of Photochemistry of the Institute of Chemistry, Chinese Academy of Sciences, developed α-Fe2O3 photoanodes that serve as a versatile and efficient oxygen atom transfer (OAT) catalyst in photoelectrochemical (PEC) cells with water molecules as the oxygen source. This OAT reaction worked via a non-radical pathway that circumvents the unselectivity caused by active radicals in traditional photo(electro)catalysis, providing a new strategy for the selective degradation of environmental pollutants and the production of value-added chemicals.
In a study published by Nature Catalysis (DOI: 10.1038/s41929-021-00659-1), the researchers reported that α-Fe2O3 photoanodes acted as an efficient OAT photocatalyst under mild conditions. A wide variety of oxygenation reactions, including thioether sulfoxidation, C=C epoxidation, Ph3P oxygenation and monooxygenation of inorganic ions (nitrite and arsenite), were investigated, which exhibited high selectivity and Faradaic efficiency.
It was proposed that the adjacent surface-trapped holes (i.e., high-valent iron oxo, FeIV=O) on α-Fe2O3 surfaces contributed to the OAT reactions. Rate law analysis of those surface-trapped holes confirmed a second-order kinetics for OAT reactions on α-Fe2O3. In contrast, it showed a first-order kinetics for OAT reactions on TiO2, which underwent a radical pathway and led to a low selectivity and Faradaic efficiency towards those OAT reactions.
Furthermore, the spin-polarized density functional theory + U study showed that the distinct surface electronic structures between α-Fe2O3 and TiO2 contributed to the distinct reaction selectivity. Photogenerated holes on α-Fe2O3 were located at those hybridized Fe 3d and O 2p orbitals, generating high-valent iron oxo (FeIV=O) that exhibited a high tendency for OAT reactions. While for TiO2, the O 2p orbitals contributed to the photogenerated holes and the produced Ti-O· initiated the unselective radical reactions.
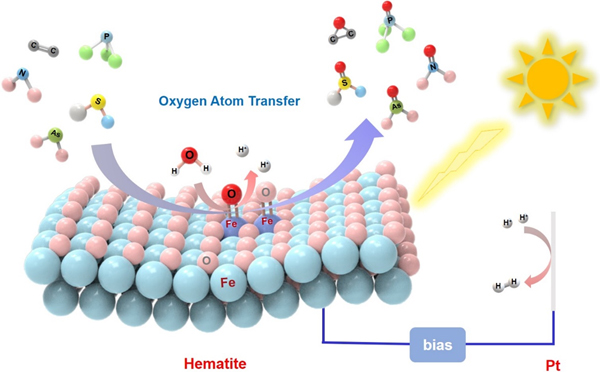
The schematic of α-Fe2O3 as a versatile and efficient oxygen atom transfer catalyst (Image by Prof. ZHANG Yuchao and Prof. CHEN Chuncheng)
Contact:
Prof. ZHAO Jincai
Institute of Chemistry, Chinese Academy of Sciences
Email: jczhao@iccas.ac.cn





