Chiral Macrocycle-Enabled Counteranion Trapping: Boosting Highly Efficient and Enantioselective Catalysis
The development of highly efficient and selective catalysis systems is one of the ultimate goals for synthetic chemists. To conquer this challenge, the enzyme-mimetic cavity of macrocyclic compounds has been exploited to promote specific transformations. Their strong binding ability can also be taken to regulate a catalytic process through binding an “indirect” reaction species. In this regard, crown-ether based cation-binding catalysis has developed into a widely-used strategy by activating and evoking anionic reaction species. On the contrary, can suitable macrocyclic receptors be applied for tightly binding a counteranion, so as to activate and influence the cation, should represent a parallel, intriguing strategy, but remains elusive.
Recently, a research group from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS), led by Prof. WANG Qiqiang, developed a chiral macrocycle-enabled counteranion trapping (MeCAT) strategy and realized very efficient and highly selective asymmetric catalysis. The work entitled “Chiral macrocycle-enabled counteranion trapping for boosting highly efficient and enantioselective catalysis” was published in Angew. Chem. Int. Ed. (2020, 59, 10894-10898).
In an earlier work, Wang’s group came up the MeCAT strategy by applying a tailor-made achiral diarylthiourea macrocycle and achieved significant acceleration in ethanedisulfonic acid-catalyzed Povarov reaction (Chem. Eur. J. 2018, 24, 4268-4272). Encouraged by the initial results, the researchers designed and synthesized a new set of BINOL-derived chiral bisthiourea macrocycles. These macrocycles possess a well-confined chiral cavity and tunable microenvironments. Binding studies suggested they were able to tightly bind disulfonates especially ethanedisulfonate anions through two cooperative thiourea sites. When applied in the Friedel-Crafts reaction of indoles with imines, just 1 mol % macrocycle in combination with 1 mol % ethanedisulfonic acid promoted excellent conversion and up to 99% ee. The acid or the macrocycle alone, however, was inactive. This catalysis system enabled an easy and even gram-scale access of a series of pre-functionalized chiral 3-indolyl methanamines with 4 or 7-halo substitution, which were otherwise inaccessible by conventional acid-catalysis. Mechanism studies revealed that the high catalytic efficiency and precise stereocontrol was ascribed to large, complexation-induced acidity enhancement and tight, directional ion-pairing triggered by the cave-like chiral macrocyclic cavity.
The developed MeCAT supramolecular catalysis strategy extends the scope of common chiral acid catalysis. It is believed to develop into a general strategy and find applications on boosting highly efficient and selective catalysis for many reactions through cationic intermediates. This work was financially supported by National Natural Science Foundation of China, and Chinese Academy of Sciences.
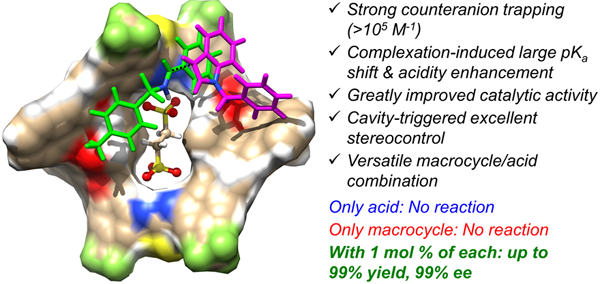
Chiral macrocycle-enabled counteranion trapping (MeCAT) for boosting highly efficient and enantioselective catalysis (Image by Prof. WANG Qiqiang)
Contact:
Prof. WANG Qiqiang
Institute of Chemistry, Chinese Academy of Sciences
Email: qiqiangw@iccas.ac.cn





