Over the past decades, N-heterocyclic carbenes (NHCs) have been demonstrated as powerful organocatalysts for the umpolung of various substrates to react with electrophiles with sp2 carbon, such as active ketones and imines. However, NHC-catalyzed reaction with electrophiles with sp3 carbon, such as simple alkyl halides, for the construction of C(sp3)-C(sp3) bond is high challenge and unsolved.
Recently, the research group led by Prof. YE Song from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) reported that the visible-light-driven NHC-catalyzed reaction with alkyl halides for the construction of C(sp3)-C(sp3) . The work entitled “Visible-Light-Driven N-Heterocyclic Carbene-catalyzed γ- and ε-Alkylation with Alkyl Radicals” was published in Angewandte Chemie (Angew. Chem. Int. Ed. 2019, 58, 18124 – 18130).
Researchers developed the merging of photoredox catalysis and N-heterocyclic carbene (NHC) catalysis for remote alkylation of enals with simple alkyl radicals. The reaction of γ-oxidized enals with alkyl halides worked well for the synthesis γ-multisubstituted-α,β-unsaturated esters, including those with challenging vicinal all-carbon quaternary centers. The synthesis of ε-multisubstituted-α,β-γ,δ-diunsaturated esters via unprecedented NHC-catalyzed ε-functionalization was also established.
This work was financially supported by National Natural Science Foundation of China, and Chinese Academy of Sciences.
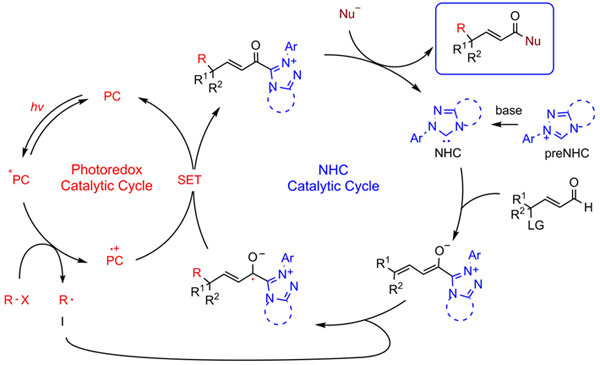
Photo/NHC-cocatalyzed remote alkylation. (Image by Prof. YE Song)
Contact:
Prof. YE Song
Institute of Chemistry, Chinese Academy of Sciences
Email: songye@iccas.ac.cn