CO2 is the major greenhouse gas. It is also a cheap, nontoxic, and abundant C1 feedstock. Efficient conversion of CO2 into valuable chemicals under mild conditions is highly desirable. However, many routes face both thermodynamic and kinetic barriers. Electroreduction is a promising approach to convert CO2 into useful products under ambient temperature and pressure, which provides future opportunity for large-scale practical application.
Electrochemical reduction of CO2 to methanol has attracted broad interest recently. However, it is very difficult to achieve satisfactory conversion rate and high selectivity of methanol simultaneously. Therefore, novel and efficient catalysts are needed to promote this field.
Recently, YANG Dexin, ZHU Qinggong and Prof. HAN Buxing from Institute of Chemistry, Chinese Academy of Sciences and their collaborators reported that copper selenide (Cu2-xSe) nanocatalysts synthesized by solvothermal method was highly efficient electrocatalyst for selective reduction of CO2 to methanol in [Bmim]PF6 (30 wt%)/CH3CN/H2O (5 wt%) ternary electrolyte.
The researchers found that the morphologies and sizes of the Cu2-xSe nanocatalysts could be regulated by varying composition of the solvents. Their performances for CO2 electroreduction depended strongly on the morphologies and sizes of the nanocatalysts which were related to the number of exposed active sites in the catalysts. Among them, Cu1.63Se exhibited high current density, Faradaic efficiency and long-term stability.
They also proposed a possible mechanism of CO2 reduction to methanol on Cu1.63Se electrode. They found that the rate-limiting step is *CO reduction to *CHO which was calculated by density functional theory. The excellent cooperative effect of Cu and Se, large electrochemistry surface area and faster electron transfer between electrolyte and Cu1.63Se surface are favorable to electroreduction of CO2.
This work supported by National Key Research and Development Program of China, National Natural Science Foundation of China, Beijing Municipal Science & Technology Commission, and the Chinese Academy of Sciences. The EXAFS experiment was conducted at Beijing Synchrotron Radiation Facility, and the result was published in Nat. Commun. (2019, 10, 677).
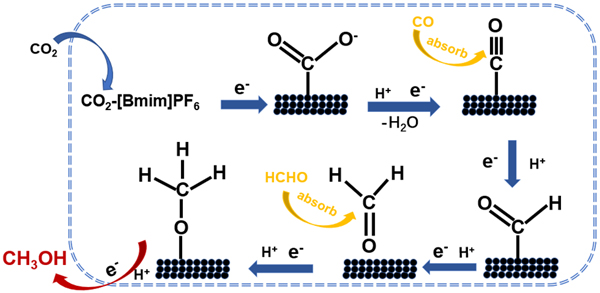
Proposed mechanism of CO2 reduction to methanol on Cu1.63Se electrode (image by Prof. HAN Buxing)
Contact:
Prof. HAN Buxing
Institute of Chemistry, Chinese Academy of Sciences
Email: hanbx@iccas.ac.cn