A Nine-atom Rhodium-aluminum Oxide Cluster Can Oxidize Five CO Molecules
Aluminum oxide materials feature good thermal and mechanical stability and it is difficult to participate directly in chemical reactions under mild conditions. Recently, scientists found that trace amounts of rhodium can promote direct participation of lattice oxygen of aluminum oxide to oxidize reactant molecules, while the fundamental mechanism of noble metal catalysis is elusive.
Recently, a research group at Institute of Chemistry, the Chinese Academy of Sciences (ICCAS) headed by Prof. HE Shenggui demonstrated that a single rhodium atom can promote the participation of five oxygen atoms to oxidize carbon monoxide molecules from a nine-atom rhodium–aluminum oxide cluster RhAl2O6+. The rhodium-aluminum oxide clusters were generated by laser ablation of a mixed-metal disk compressed with rhodium and aluminum powders, and the generated RhAl2Om+ clusters were mass-selected, confined, and then interacted with CO in an ion trap reactor under mild conditions. On the interaction of RhAl2O6+ with CO, the consecutive transfer of five oxygen atoms to CO has been unexpectedly identified. This work was published recently on Nature Communications.
This result is a sharp improvement in the field of cluster science. It was previously reported that a single noble metal atom doped heteronuclear oxide cluster can transfer at most two oxygen atoms to oxidize CO molecules. Density functional theory calculated results demonstrated that the rhodium atom functions as the trapping site to anchor and oxidize CO molecules by the oxygen atoms connected with rhodium. Furthermore, rhodium atom acts as the primarily oxidative center to accumulate the large amounts of electrons that stored originally in the removed oxygen atoms, then the polarity of rhodium is ultimately transformed from positive to negative. This gas-phase study sheds new insights into the nature of noble metal catalysis that takes place in the related condensed phase.
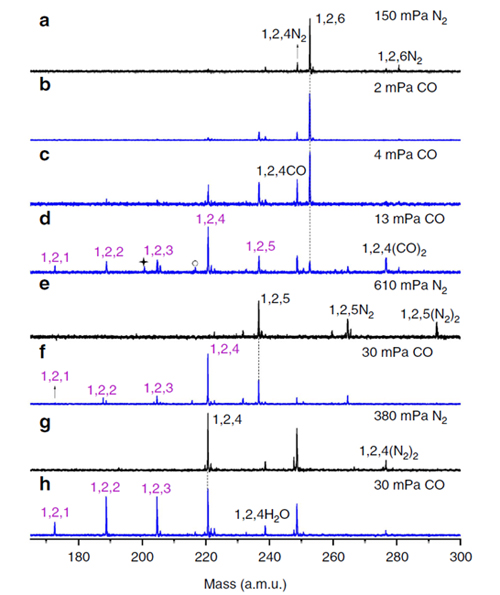
Reactivity of RhAl2O4-6+ clusters with CO (Image by HE Shenggui)
Contact:
Prof. HE Shenggui
The Laboratory for Structural Chemistry of Unstable Species ,
Institute of Chemistry, Chinese Academy of Sciences
Email: shengguihe@iccas.ac.cn





