Two-State Reactivity in Low-Valent Iron-Mediated C–H Activation
As a representative earth-abundant first-row transition metals, iron is the most common transition metal in earth’s crust. C–H bond activation/functionalization promoted by low-valent iron complexes has recently emerged as a promising approach for the utilization of earth-abundant first-row transition metals to carry out this difficult transformation. However, mechanism concerning this C-H activation by low-valent iron is still very unclear, which definitely hampers further development of this transformation.
Research group led by Prof. CHEN Hui in Institute of Chemistry, Chinese Academy of Sciences (ICCAS), made progress in the mechanistic study of C–H bond activation/functionalization promoted by low-valent iron. By employing combined DFT and high level ab initio coupled cluster computational approach, they for the first time uncovered that two-state reactivity (TSR) is operative in C–H bond activation promoted by low-valent iron species. This work was published on J. Am. Chem. Soc. 138, 3715-3730.
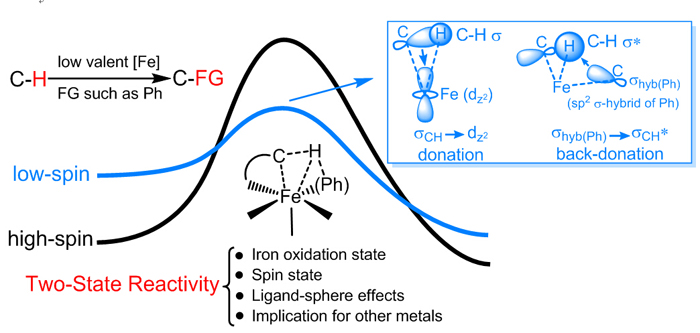
TSR scenario in C-H bond activation by low-valent iron species. (Image by CHEN Hui)
In the corresponding research field of inorganic and bioinorganic chemistry, TSR is a well-known and widely accepted concept for C-H bond activation promoted by high-valent iron via hydrogen abstraction mechanism, which features a advantageous reactivity in high-spin state over low-spin one. In organometallic chemistry, however, C-H bond activation promoted by low-valent iron is revealed to be via σ-bond metathesis mechanism, in which low-spin singlet state is found to have superior reactivity over the high-spin one. Based on this new TSR scenario, the ligand effect was well explained for C–H bond activation/functionalization promoted by low-valent iron. In addition, upon enough ligand stabilization, researchers predicted that in besides iron, manganese is another candidate for potential usage of its low spin state to bring about efficient C–H bond activation.
This work is financially supported by ICCAS and National Natural Science Foundation of China.
Contact:
Prof. CHEN Hui
Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences
Email: chenh@iccas.ac.cn





