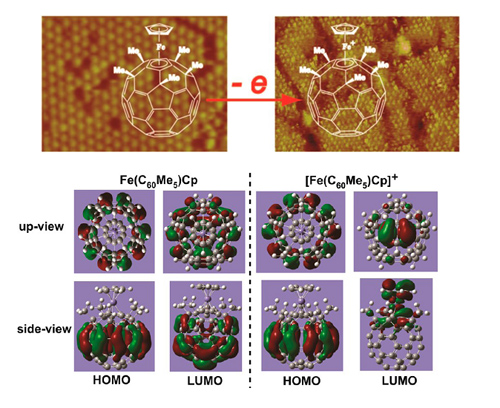
The electron donor−acceptor dyads are an emerging class of materials showing important applications in nonlinear optics, dye-sensitized solar cells, and molecular electronics. Investigation of their structure and electron transfer at the molecular level provides insights into the structure−property relationship and can benefit the design and preparation of electron donor−acceptor dyad materials. Herein, the interface adstructure and electron transfer of buckyferrocene Fe(C60Me5)Cp, a typical electron donor−acceptor dyad, is directly probed using in situ electrochemical scanning tunneling microscopy (STM) combined with theoretical simulations. It is found that the adsorption geometry and assembled structure of Fe(C60Me5)Cp is significantly affected by the electrochemical environments. In 0.1 M HClO4 solution, Fe(C60Me5)Cp forms well-ordered monolayers and multilayers on Au(111) surfaces with molecular dimer as the building block. In 0.1 M NaClO4 solution, typical six-fold symmetric close-packed monolayer with vertically adsorbed Fe(C60Me5)Cp is formed. Upon electrochemical oxidation, the oxidized Fe(C60Me5)Cp shows higher brightness in an STM image, which facilitates the direct visualization of the interfacial electrochemical electron transfer process. Theoretical simulation indicates that the electrode potential-activated, ne-electron transfer from Fe(C60Me5)Cp to the electrode leads to the change of the delocalization character of the frontier orbital in the molecule, which is responsible for the STM image contrast change. This result is beneficial for understanding the structure and property of single electron donor−acceptor dyads. It also provides a direct approach to study the electron transfer of electron donor−acceptor compounds at the molecular level.
J. Am. Chem. Soc., 2014, 136 (8), 3184–3191