Oxygen Radical Chemistry Probed by Study of Atomic Clusters
Oxygen radicals are typical of important reactive intermediates in the atmospheric, chemical, and biological processes. It is difficult to capture and study oxygen radicals. Recently, under the supports from the National Natural Science Foundation of China, the Chinese Academy of Sciences (CAS), and the Ministry of Science and Technology, Dr. He Shenggui and his co-workers from the State Key Laboratory for Structural Chemistry of Unstable and Stable Species in the Institute of Chemistry CAS proposed a new strategy to study oxygen radical chemistry. The strategy is to prepare oxygen radicals onto atomic clusters. The cluster structures can be well determined, which permits rather detailed understanding of the bonding and reactivity of oxygen radicals by tuning the cluster compositions, sizes, electronic structures, etc. The research progress on C-H bond activation by oxygen-centered radicals has been summarized in Acc. Chem. Res. 2012, 45, 382.
To study the oxygen radical chemistry with the cluster approach, the researchers defined a number D that can be termed as oxygen deficiency to classify metal oxide clusters into different types (Theor. Chem. Acc. 2010, 127, 449). The experimental and theoretical studies indicated that the type of clusters with D=1 contain oxygen-centered radicals as active centers to react with very stable molecules (such as methane, Chem. Commun. 2010, 46, 1736) at room temperature. The single electron transfer in the process of molecular oxygen adsorption is an important way to generate oxygen radicals barrierlessly (J. Phys. Chem. A 2010, 114, 10024; 2011, 115, 10245). The oxygen radicals in a series of heteronucelar oxide clusters are found to be bonded with main group elements aluminum (Chem. Eur. J. 2011, 17, 3449), silicon (J. Phys. Chem. C 2010, 114, 12271; Chem. Eur. J. 2010, 16, 11463 / VIP article), and phosphorus (Phys. Chem. Chem. Phys. 2010, 12, 12223) rather than with transition metal vanadium. The local spin density distribution (Phys. Chem. Chem. Phys. 2010, 12, 3984; J. Phys. Chem. C. 2011, 115, 13329) and local charge distribution (Chem. Eur. J. 2011, 17, 11728) around the oxygen radicals on the clusters can influence the reaction rate constants with carbon monoxide and alkane molecules dramatically. These findings are fundamental basis to tune and control the chemical reactivity and selectivity of oxygen radicals with stable small molecules.
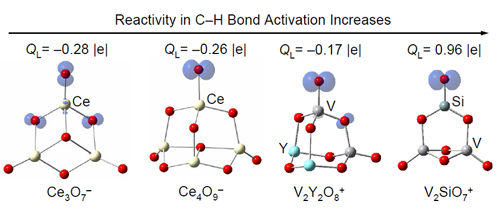
Figure 1 The local spin density distribution and local charge distribution around the oxygen radicals can be tuned to control their chemical reactivity. (Imaged by He Shenggui and Ding Xunlei)





