Single Molecule Imaging Reveals Transforming Growth Factor-b-induced Type II Receptor Dimerization
Wei Zhang, Yaxin Jiang, Qiang Wang, Xinyong Ma, Zeyu Xiao, Wei Zuo. Xiaohong Fang*, Ye-Guang Chen*
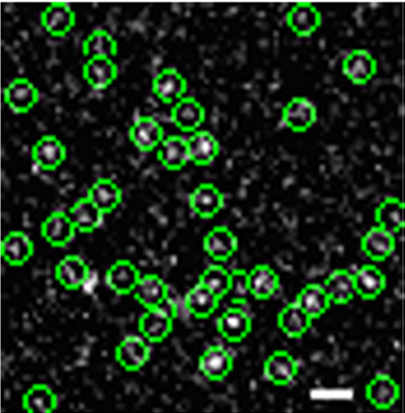
We present in this paper our new observation of transforming growth factor receptor monomer dimerization. Transforming growth factor-beta (TGF-b) signaling through its type I (TbRI) and type II (TbRII) receptors controls many crucial cellular processes such as cell proliferation, differentiation, motility and apoptosis.It is generally believed that the initial receptor dimerization is an essential event for receptor activation. However, the previous studies suggested that TGF-b signals by binding to the pre-existing TbRII homodimer. Here, using single molecule microscopy to image green fluorescent protein (GFP)-labeled TbRII on the living cell surface, we discovered the existence of individual TbRII molecules on the cell membrane. We further investigated the oligomeric status of TbRII both at resting state and after TGF-b1 treatment in living cells, and found for the first time that the monomeric TbRII are dimerized upon ligand stimulation.
Our results reveal an unrecognized model in which the activation of serine-threonine kinase receptors is also accomplished via monomer dimerization upon ligand binding. The results should be of wide interest to researchers in biophysics, biochemistry and cell biology.
Proc Natl Acad Sci USA, 2009, 106,15679-15683.